Fertilisers in France in the nineteenth century
PDF
In the nineteenth century, farmers used organic soil conditioners and/or fertilisers in varying proportions. In Western Europe, agricultural yields were insufficient to guarantee food security for the population. However, this was a century in which the use of fertilisers, particularly chemical fertilisers developed from the 1840s onwards, developed and allowed, among other things, to an increase in agricultural production, particularly cereals. How can a historical approach help us understanding the evolution of the fertilisation practices that we have inherited? The aim of this article is to show how agronomists rationalised soil fertilisation techniques by adapting to the emergence of agrochemicals in the nineteenth and early twentieth centuries.
- 1. Definitions and global reflections
- 2. Emergence of agricultural chemistry and knowledge of plant nutrition
- 3. The interest of agronomists in fertilisers
- 4. Agricultural reuse of urban wastewater
- 5. Fighting fraud
- 6. Late 19th-early 20th century: superphosphates and chemical nitrogen fertilisers
- 7. Messages to remember
1. Definitions and global reflections
During the nineteenth century, the technical vocabulary used for fertilisers was still vague, both among agronomists and farmers, and it was not uncommon to find confusion between soil conditioner and organic fertiliser [1]. Agronomists are educated, subjective observers of farming practices, often members of academic societies, whose aim is to propose techniques for improving agricultural productivity to ensure food security for the population [2]. Those who work the land, who handle the plough and other farming tools, are usually called peasants.


As far as so-called organic fertilisers are concerned, some farmers use farmyard manure (Figure 1) and green manure, i.e. crop residues left in the field and buried when ploughing (Figure 2). To this can be added the columbine, i.e. droppings from dovecotes and aviaries.

These chemical fertilisers enriched agricultural soils with the three main fertilising minerals: nitrogen, phosphoric acid or calcium phosphate and potash. However, the role and benefits of chemical fertilisers were the subject of fierce debate and controversy among agronomists, mainly French, in the second half of the 19th century, at least until the 1880s [11]. During the nineteenth century, agronomic discourse on fertilisers became complex. Some discourses praised the virtues of chemical fertilisers and denigrated farming routines. On the other hand, others rejected chemical fertilisers and promoted organic manure. Finally, a third, more moderate, set of arguments closely linked the promotion of both chemical fertilisers and organic fertilisers.
2. Emergence of agricultural chemistry and knowledge of plant nutrition


Liebig, a German chemist and agronomist, is one of the founders of organic chemistry. His countless publications have been translated into many languages and have often been republished. A pupil of Thénard and Gay-Lussac, and supported by Humboldt, he defended his thesis in Giessen, where he became a professor in 1824 (in 1852, Liebig was appointed professor in Munich). The laboratory he directed became a centre for training and research in the field of chemistry.

Liebig’s “Chimie organique appliquée à la Physiologie végétale et à l’Agriculture” (Figure 6) was published in September 1840. This text was at the origin of the use of chemical fertilisers and the development of their industry. The book was a reworking of a text published in French four months earlier, as an introduction to his “Traité de Chimie Organique” (April 1840, Fortin et Masson, Paris). Agricultural chemistry and plant physiology help us to understand the importance of mineral inputs for soil fertility.
Liebig criticised the humus theory, defended at the time by many of the most influential European agronomists of the early 19th century. This theory was set out by Albrecht Thaër (1752-1828) in his “Principes raisonnés d’Agriculture” in the early 1810s, in which he explained that humus, i.e. the brown matter that forms in the soil after organic matter (now known as soil organic matter) has been broken down by the action of oxygen, provides plant nutrition. In his 1840 work, “La Chimie dans ses rapports avec l’Agriculture et la Croissance des Plantes “(Chemistry in its relation to agriculture and plant growth), Liebig demonstrated that the real plant nutrients were the mineral products derived from the decomposition of humus and not humus itself, as the proponents of the humus theory believed [15]. Liebig invalidated this theory which led to its definitive rejection.

In “La Chimie Organique appliquée à l’Agriculture et à la Physiologie” (Organic chemistry applied to agriculture and physiology) and the “Traité de Chimie Organique” (Treatise on organic chemistry), Liebig formulated the theory of the mineral nutrition of plants and criticised the theory of humus, formulating two laws at the same time:
- The law of restitution (the elements taken from the soil during harvesting must be returned to it through the application of soil improvers or fertilisers).
- The law of the minimum (Figure 8): “The yield of a crop is determined by the element that is present in the smallest quantity in relation to the needs of the crop” (Liebig). This law explains that plant growth is limited by the assimilable element present in the environment in the lowest concentration (also known as the limiting factor). Liebig then explained that this limiting factor (mainly nitrogen, as it was thought in the 19th century, whereas today we know that it is phosphorus that is the limiting factor) must be compensated for by applying fertiliser (organic and/or chemical). (See How to feed plants while polluting less?)
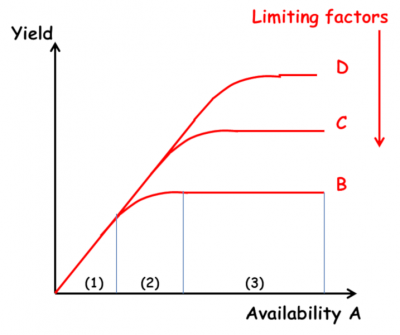
The importance of the law of the minimum in agronomy played a role in the excesses of ultra-productivist agriculture after the Second World War, by encouraging over-fertilisation. Marika Blondel-Mégrelis explains, however, that a closer reading of Liebig does not make him the father of intensive agriculture, but rather the precursor of organic farming [18]. Gilles Lemaire believes that Liebscher and his law of the optimum set out in 1885 is more relevant: “each nutrient is used all the more efficiently if the availability of the most limiting element is brought close to its optimum”.
Liebig was not the first to state the law of the minimum; Sprengel had already formulated it in 1828. De Saussure too, as early as 1804, announced the mineral nutrition of plants. And yet it was Liebig’s work and results that remained at the forefront of the scientific scene in the 19th century, and that the history of science continues to highlight [19].
However, these proposals gave rise to long and tenacious debates and controversies. In France, for example, the agronomists Jean-Baptiste Dumas (1800-1884) and Jean-Baptiste Boussingault (1802-1887) took a very long time to accept the theory of mineral nutrition and especially the importance of phosphorus and potassium. So why did Liebig remain so prominent in the history of agrochemistry, to the detriment of Sprengel, de Saussure and Liebscher? Marika Blondel-Mégrelis offers the convincing explanation that Liebig was very busy with his research and promoting it, an activity in which he excelled. While others were more or less thwarted in their careers or preoccupied with teaching duties. It was his effectiveness in disseminating his ideas and his capacity for polemic that enabled Liebig to make his mark and leave some of his colleagues in the shade. This overshadowing became increasingly relative as environmental concerns about agriculture led to the reappearance of proposals that rivalled Liebig’s, such as Liebscher’s law of optimum yield.
3. The interest of agronomists in fertilisers

In the 19th century, agronomists devoted a great deal of their writings to the question of fertilisers. Adrien de Gasparin (1783-1862; Figure 9) wrote 153 pages on nitrogen fertilisers in his « Cours d’agriculture » of 1843 [21].
Jean-Baptiste Boussingault (1802-1887; Figure 10), for his part, became interested in converting saltpetre into fertiliser in the early 1860s [22]. Saltpetre was used primarily to make gunpowder, but not exclusively for military purposes. At the time, agronomists were on a ” veritable hunt for fertilizers ” (Jean Boulaine’s expression). This is why some seek to use the fertilising properties of “nitre” or saltpetre for agricultural purposes. This is potassium nitrate, the whitish crystals that form in damp places, somewhat different from the sodium nitrate found in Chile and Peru.

4. Agricultural reuse of urban wastewater

Dehérain explained that wastewater could only be used by farmers if it was largely diluted. Hence the small market for this wastewater, which could only be bought by farmers who had easy access to large quantities of water.
In most towns, the fixed sewage collection pits used to collect wastewater were often leaky. In the Hôtel de Ville area of Paris, for example, up to 34.3 milligrams of ammonia (probably mg/l but the author does not specify this) were measured, when the normal quantity varied between 0.03 mg and 0.06 mg/l [25]. The contents of the pits were therefore infecting the French capital’s drinking water supplies. This was a real danger: the link had already been made between human faeces and the transmission of cholera, which was so devastating in 19th-century cities [26]. Dehérain therefore proposed abandoning the stationary pits, which he described as “hotbeds of infection” [27], and suggested using the sewage emptying system adopted in London as a model.
To assess the benefits of using sewage water to irrigate farmland, an experimental field was set up in Gennevilliers (without considering the protests of the inhabitants) from June 1869. According to Dehérain, the experiment was so conclusive that, by 1870, around fifty farmers were irrigating their fields with Parisian sewage. After the 1870 war and the Commune of 1871, the resumption of irrigation with sewage led to an increase in the price of renting farmland in the Gennevilliers area: it tripled in 1872, rising from 80 francs per hectare to 250 francs.
Irrigation of meadows by completely immersing them in sewage water was successfully practised in Edinburgh. Yields were high and fodder selling prices are high, which benefited the farmers. What’s more, the chemical analyses cited by Dehérain show that this practice limits pollution of river water and the sea. In 100 kg of Edinburgh’s wastewater, there were 11.445 g of nitrogen before it was spread on the meadows. Only 2.320g of nitrogen was found in the water that flowed into the sea. Of course, the analysis methods used at the time did not allow for very detailed measurements, and leached water was not included in the analysis. Agronomic studies have demonstrated the long-term nature of water contamination by nitrates and other substances such as atrazine by studying leaching and the adsorption/desorption characteristics of water in agricultural soils. However, Dehérain noted, during experiments carried out at Lodge-farm near London, that “nitrates, which do not exist in sewage water, are on the contrary found in notable proportions in liquids which have passed through arable soil”: from the end of the 1860s and the beginning of the 1870s, it was known that nitrates were filtered very slowly by agricultural soils. But the harmfulness of this was not yet known.
Irrigating meadows with urban wastewater was therefore seen as a filtering technique that enabled water to be discharged into the sea or into watercourses that had become “clean” or almost “clean” (in all cases, according to the state of knowledge at the time).
5. Fighting fraud

With the development of the “animal dung” market in Nantes from the mid-1820s onwards, fraud increased, fleecing many farmers who were swindled by unscrupulous merchants. To combat this fraud, a fertiliser analysis laboratory was set up in the Loire-Inférieure departement in 1851, run by chemist Pierre-Adolphe Bobierre (1823-1881).

Agronomic stations were born on the other side of the Rhine. These were research structures dedicated to agrochemistry and mainly to the control of chemical fertilisers. Grandeau was convinced that the agronomic station and its experimental field, based on the German model, was the most appropriate and effective structure for research and liaison with the agricultural world.

The question of the real value of the fertilising elements found in the fertilisers purchased by farmers was raised at the agronomy stations. Often, farmers bought their fertilisers empirically, based on the appearance or smell of the substance offered to them. This approach was considered rudimentary and rejected by agronomists. All too often, during the 1860s and 1870s, the names of fertilisers were misleading, while the figures supposed to indicate their nutrient content were vague and unreliable.

The spread of technical and practical knowledge about the agricultural use of fertilisers remained limited, even though the quantities of fertilisers used tended to increase at the end of the 19th century, probably more on large capitalist farms than on small farms. At the beginning of the 20th century, farmers were using between 20 and 100 tonnes of manure per hectare, depending on the size of the farm. However, in the 1870s, agronomists were still hotly debating the standards to be set and the methods of analysis to be standardised. Sometimes there were major discrepancies between two analyses of the same fertiliser, which resulted in the price varying by several francs/kg, a considerable variation on a tonne scale. In his “Traité d’analyse des matières agricoles“, published in 1877, Grandeau gave nitrogen, phosphorus and potassium values that could be used to determine the quality of a mineral fertiliser using chemical analysis. But there is still no scientific consensus on these values.
6. Late 19th-early 20th century: superphosphates and chemical nitrogen fertilisers
In France in the 19th century, unlike in England, agronomists were highly critical of superphosphates: a chemical fertiliser obtained by treating naturally occurring phosphates, such as “animal dung”, with sulphuric or phosphoric acid. Hence the massive use of organic urban waste as agricultural fertiliser. It was not until the 1870s that their reluctance faded.
 The use of superphosphates (Figure 17), which could be used on all types of soil, became widespread at the beginning of the 20th century, as did phosphorus-removal slag, i.e. the leftovers from the treatment of cast iron using the Gilchrist-Thomas process. According to Grandeau, French farmers used around 40 kg/ha of phosphate fertiliser around 1899-1900. These phosphate fertilisers became indispensable to agriculture. In the 20th century, agronomists quickly discovered that phosphorus was a non-renewable resource. This is why animal and human waste continues to be used to fertilise agricultural soils with phosphate fertilisers [34].
The use of superphosphates (Figure 17), which could be used on all types of soil, became widespread at the beginning of the 20th century, as did phosphorus-removal slag, i.e. the leftovers from the treatment of cast iron using the Gilchrist-Thomas process. According to Grandeau, French farmers used around 40 kg/ha of phosphate fertiliser around 1899-1900. These phosphate fertilisers became indispensable to agriculture. In the 20th century, agronomists quickly discovered that phosphorus was a non-renewable resource. This is why animal and human waste continues to be used to fertilise agricultural soils with phosphate fertilisers [34].
Potash and synthetic nitrogen began to be used after 1914, but the scientific advances that enabled them to be used to their full potential came later. The production of chemical nitrogen fertilisers had to wait until ammonia was synthesised in 1913. These synthetic chemical fertilisers made it less essential to use powders or water from urban cesspits.
The history of fertiliser agronomy in the 19th century is therefore above all a history of the emergence of agrochemicals, which took hold in the last third of the century and at the beginning of the 20th century. At the time, agronomists were not concerned about possible pollution linked to the use of fertilisers, even though pollution from industrial sources had already been identified and, in some cases, denounced [35].
7. Messages to remember
- From the mid-nineteenth century onwards, chemical fertilisers were used to supplement organic fertilisation.
- The first half of the nineteenth century in particular saw the emergence of agricultural chemistry and plant physiology, making it possible to understand the importance of mineral inputs for soil fertility.
- However, the spread of technical and practical knowledge about the agricultural use of fertilisers remained limited, even though the quantities of fertilisers used tended to increase at the end of the 19th century.
- In the nineteenth and early twentieth centuries, agronomists did not express any concerns about possible pollution linked to the use of fertilisers, even though pollution from industrial sources had already been identified and, in some cases, denounced.
Notes and references
Cover image. The kelp gatherers, Paul Gauguin (1889), Essen, Museum Folkwang, Allemagne – Public domain, licence CCO
[1] This leaflet is an abridged and rewritten version of the following article: Knittel F. (2017), « Agronomie des engrais en France au XIXe siècle. Salpêtre, déchets urbains, engrais chimiques : trois exemples de valorisation agricole », Histoire et Sociétés Rurales, n° 48, p. 177-200 – (in French).
[2] Knittel F. (2009), Agronomie et innovation. Le cas Mathieu de Dombasle (1777-1843), Nancy, Presses Universitaires de Nancy – (in French).
[3] Marling or liming corrects the acidity of cultivated soils.
[4] Bakels, C. (1997), « The beginnings of manuring in Western Europe », Antiquity, n°71, p. 442-445.
[5] Lachiver M. (2006), Dictionnaire du monde rural : les mots du passé, Paris, Fayard, [1er éd. 1997], p. 1050 – (in French).
[6] Moriceau J.-M. (2002), Terres mouvantes, Paris, Fayard, p. 264-265 – (in French).
[7] Lecouteux E. (1879), Cours d’économie rurale, Paris, p. 215-217 – (in French).
[8] Bourrigaud R. (1993), Le développement agricole au XIXe siècle en Loire-Atlantique. Essai sur l’histoire des techniques et des institutions, Thèse de droit, Uni. de Nantes, dactyl., p. 227-263 – (in French).
[9] Lachiver M., supra note 5 (p. 533 et p. 57) – (in French).
[10] Mazoyer M., Roudart L. (2002), Histoire des agricultures du monde, Paris, Seuil, [1er éd.1997], p. 85 – (in French).
[11] Boulaine J. (2006), « Histoire de la fertilisation phosphatée, 1762-1914 », Etude et Gestion des Sols, n°2, p. 129-137 – (in French).
[12] Jas N. (2001), Au carrefour de la chimie et de l’agriculture, les sciences agronomiques en France et en Allemagne, 1840-1914, Paris, EAC – (in French).
[13] Mathieu de Dombasle, C.-J.-A. (1828), “Examen critique des éléments de chimie agricole de M. H. Davy“, Annales de l’agriculture française, 1820, reprinted in Annales Agricoles de Roville, tome 5, p. 134-197 – (in French).
[14] Blondel-Mégrelis M., Robin P. (2002), « 1800 et 1840. Physiologie végétale et chimie agricole. Liebig, une fondation à questionner », dans Belmont A. (dir.), Autour d’Olivier de Serres. Pratiques agricoles et pensée agronomique du Néolithique aux enjeux actuels, Rennes, Presses Universitaires de Rennes, p. 275-296 – (in French).
[15] Feller C., Angers D., Chenu Cl., (2023), « Histoire d’humus, 2. De la « théorie de l’humus » à la théorie minérale », Les mots de l’agronomie – (in French).
[16] Robin P., Blondel-Mégrelis M. (2001), « Physiologie végétale chimique et chimie agricole, 1800-1840. Saussure, une publication à ressusciter », Comptes rendus de l’Académie d’agriculture de France, 87-4, p. 31-59 – (in French).
[17] Lemaire G. Tang L., Bélanger G., Zhu Y., Jeuffraoy M.-H (2021), « Forward new paradigms for crop mineral nutrition and fertilization towards sustainable agriculture », European Journal of Agonomy, 125.
[18] Blondel-Mégrelis M. (2020), « Liebig et la loi du minimum », Les mots de l’agronomie – (in French).
[19] Liebscher cité par Lemaire G., (2019), « Une nouvelle approche de la fertilisation des cultures », Agronomie, environnement et sociétés, Vol. 9-1, p. 56 – (in French).
[20] Boulaine J. (1996), Histoire de l’agronomie en France, Paris, Tec et doc éd., 2e éd., p. 251 – (in French).
[21] Gasparin A. de (1843), Cours d’agriculture, Paris, Librairie de la Maison rustique, p. 506-606 – (in French).
[22] Boussingault J.-B. (1861), Agronomie, chimie agricole et physiologie, Paris, Mallet-Bachelier, 2e éd, tome 2 – (in French).
[23] Boulaine J. (1994), « Jean-Baptiste Boussingault (1802-1887) : Professeur d’agriculture (1845-1848), de chimie agricole (1851-1887) », dans Fontanon Cl., Grelon A. (dir.), Les professeurs du Conservatoire national des arts et métiers, Paris, INRP/CNAM, tome 1, p. 246-258 – (in French).
[24] Pierre-Paul Dehérain teached chemistry at the Grignon agricultural college.
[25] Dehérain P.-P. (1873), Cours de chimie agricole, Paris, Hachette, p. 488 – (in French).
[26] Bourdelais P., Raulot J.-Y. (1987), Une peur bleue, Histoire du choléra en France, Paris, Payot – (in French).
[27] Dehérain P.-P., note 23, p. 488 – (in French).
[28] Benoît M., Knittel F., Cussenot M. (2001), « Trois moments-clés de l’agronomie en Lorraine au XIXe siècle », dans Clément J.-Fr., Le Tacon Fr. (dir.), Sciences et techniques en Lorraine à l’époque de l’Ecole de Nancy, Nancy, M. J. C. Pichon éd., p. 225-239 – (in French).
[29] At the same time, from 1871 until 1888, Grandeau held the chair of agriculture at the Ecole Nationale Forestière, founded in Nancy in 1824.
[30] Knittel F., Rollet L. (2016), « Louis Grandeau (1834-1911), professeur de chimie agricole et de physiologie appliquée à l’agriculture », Rollet L., Bolmont E., Birck Fr., Cussenot J.-R. (dir.), Les enseignants de la Faculté des sciences de Nancy et de ses instituts. Dictionnaire biographique (1854-1918), Nancy, PUN-Editions Universitaires de Lorraine, p. 270-274 – (in French).
[31] Benoît M., Knittel F., Cussenot M. (2001), « Trois moments-clés de l’agronomie en Lorraine au XIXe siècle », dans Clément J.-Fr., Le Tacon Fr. (dir.), Sciences et techniques en Lorraine à l’époque de l’Ecole de Nancy, Nancy, M. J. C. Pichon éd., p. 225-239, p. 236 – (in French).
[32] Grandeau L. N. (1878), Champs d’expérience de la station agronomique de l’Est. Essai de culture de 1870 à 1877, Paris, Berger-Levrault – (in French).
[33] Roussille A. (1879), « La fraude dans le commerce des engrais. Les phospho-guanos », Journal d’Agriculture Pratique, n°1, p. 562-563 – (in French).
[34] Schipanski, M. E., Bennett, E. M. (2011), « The influence of agricultural trade and livestock production on the global phosphorus cycle », Ecosystems, n°15, p. 256-268.
[35] Le Roux Th. (2011), Le laboratoire des pollutions industrielles. Paris, 1770-1830, Paris, Albin Michel – (in French).
The Encyclopedia of the Environment by the Association des Encyclopédies de l'Environnement et de l'Énergie (www.a3e.fr), contractually linked to the University of Grenoble Alpes and Grenoble INP, and sponsored by the French Academy of Sciences.
To cite this article: KNITTEL Fabien (May 26, 2024), Fertilisers in France in the nineteenth century, Encyclopedia of the Environment, Accessed December 22, 2024 [online ISSN 2555-0950] url : https://www.encyclopedie-environnement.org/en/life/fertilisers-france-nineteenth-century/.
The articles in the Encyclopedia of the Environment are made available under the terms of the Creative Commons BY-NC-SA license, which authorizes reproduction subject to: citing the source, not making commercial use of them, sharing identical initial conditions, reproducing at each reuse or distribution the mention of this Creative Commons BY-NC-SA license.







