A carbon cycle disrupted by human activities
PDF
All living beings are built from carbon atoms. These are extracted from atmospheric CO2 by plants, algae and certain bacteria, using solar energy: this is photosynthesis. The respiration and decomposition of living beings release this CO2 back into the atmosphere. In addition to this short life cycle, there is a slow geological cycle that stores carbon in the form of limestone and fossil hydrocarbons. Limestone comes from the shells of marine organisms while hydrocarbons are formed by burial of organic sediments. The combustion of fossil resources currently represents a short circuit from this slow cycle to the short cycle that largely dominates natural regeneration processes. This leads to a rapid accumulation of CO2 in the atmosphere, causing global warming, and ocean acidification that can disrupt marine life.
1. The origins
Carbon is a fairly abundant element produced -like most light elements in the core of stars- by nuclear fusion of hydrogen (see Nuclear Energy). The carbon on Earth and the other planets comes from a star that exploded before the formation of the Solar System. During such an explosion (supernova) the condensable matter formed clouds of gas and dust which then aggregated to form planets in the early Solar Nebula. Like all non-radioactive elements [1], this initial carbon is permanently conserved and recycled since the formation of the Earth, passing through different environments and combining with different molecules.
This primitive material, prior to the formation of the Earth, has reached us almost intact in some meteorites called carbonaceous chondrites. It is also found in comets. Remarkably, carbon forms various elementary organic chemical compounds, such as hydrocarbons, alcohols and amino acids (see How to study the organic molecules of comets). Giant planets like Jupiter or Saturn are composed mainly of hydrogen (90%) and helium (nearly 10%), but carbon is also present (0.1%) in the form of methane (CH4) or other hydrocarbons.
On rocky planets like the Earth, the high temperatures during their formation have led to the separation of the metallic elements (iron and nickel) that form the core of the less dense silicates that make up the mantle. It is estimated that carbon has been distributed in comparable proportions between nucleus [2] and mantle. In the latter, pure carbon subjected to high pressure and temperature crystallizes into diamonds: they are found on the surface in former highly active volcanic pipes, where rapid cooling has blocked any change in state. However, it is mainly present in the form of carbonates constituting between 0.003 and 0.03% of the mass of the mantle. It is released into the atmosphere as carbon dioxide, CO2, during volcanic eruptions. On Mars and Venus, volcanism also emits CO2, which is the main component of the atmosphere. CO2 was also one of the main components of the Earth’s atmosphere before photosynthesis appeared, which released oxygen while fixing atmospheric carbon as organic matter (see The biosphere, a major geological player).
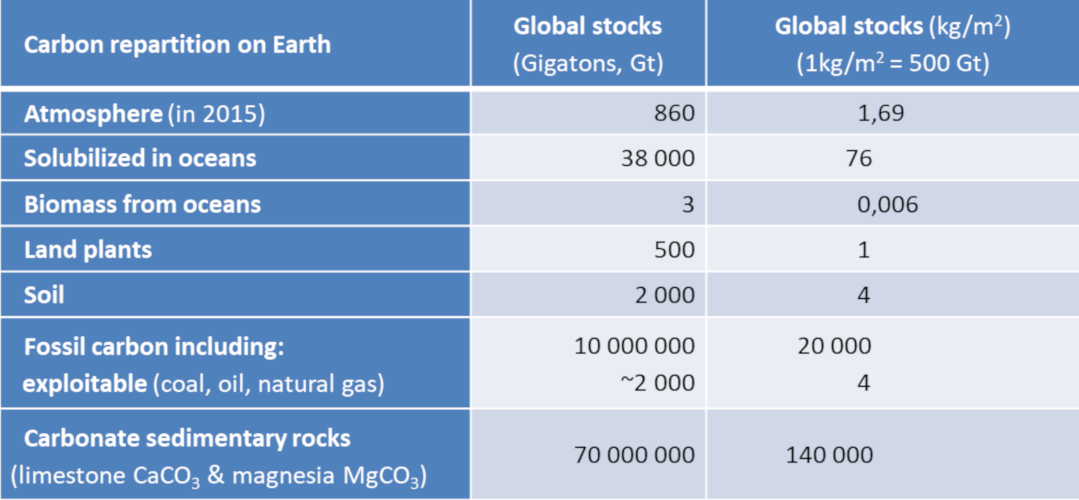
2. Earth carbon reservoirs
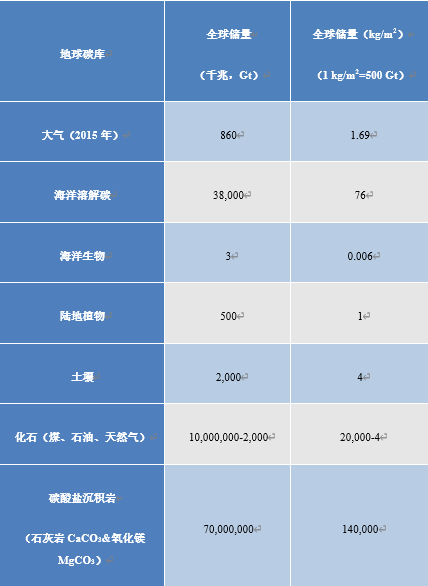
Carbon is stored in different forms in media called reservoirs, the main ones are listed in Table 1. These reserves are expressed in gigatonnes of carbon (1GtC = 1012 kg of carbon). More intuitively, these quantities can be reported on the total surface area of the globe (510 million km2 or 510 x 1012 m2), so that 1 kg/m2 corresponds to a total of 510 GtC. The atmosphere currently contains 1.69 kg/m2 of carbon in the form of CO2 well mixed by the winds. This quantity can easily be deduced [3] from the atmospheric CO2 concentration, currently 400 ppmv (one ppmv indicates a proportion of 10-6 by volume, or the number of molecules). And a 1 ppmv increase corresponds to 2.12 GtC.
The ocean contains nearly 50 times more carbon than the atmospheric reservoir. The dissolved CO2 reacts with water to give carbonic acid H2CO3, itself in equilibrium with the hydrogen carbonate ion HCO3– (also called bicarbonate) and the carbonate ion CO32-. The HCO3– form represents nearly 95% of ocean carbon. The ocean-atmosphere exchange transfers CO2 either in one direction (atmosphere to ocean) or in the other (ocean to atmosphere), tending towards a temperature-dependent balance of concentration ratios.
The total mass of living oceanic organisms (called ocean biomass) contains a more limited amount of carbon, 6 g/m2, mainly in the form of plankton and bacteria. Terrestrial vegetation represents 1 kg/m2 of carbon, while 4 kg/m2 is stored in the organic debris of the soil (constituting humus). These are averages relative to the total surface area of the globe.
Table 1. Carbon reservoirs (mantle and terrestrial core are not mentioned due to high uncertainties and limited interactions with the environment).

Another part of the carbon, estimated at 20 t/m2, is stored as fossil carbon of organic origin (unoxidized). This carbon is found mainly in sediments and sedimentary rocks, in very low concentrations (kerogens). A small portion evolves into methane (CH4), which can be trapped on the ocean floor and in frozen soils as hydrates. Only a small fraction of the fossil carbon (less than 0.01%) has been concentrated, matured and sequestered to produce exploitable deposits of hydrocarbons (gas and oil) and coal (see Oil: evidences for its biological origin).
At time scales of hundreds of millions of years, sediments are carried away by tectonic plate movements and partially integrate into the mantle, whose total carbon reserve, very poorly known, is estimated to be even higher than that of limestone rocks.
3. Flows disrupted by human activity
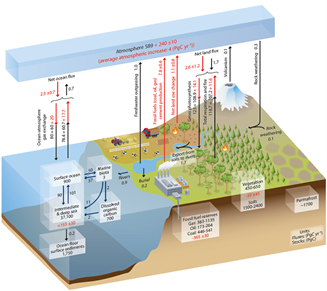
The carbon flows between the different reservoirs are illustrated in Figure 2 (taken from [4]) which distinguishes between the pre-industrial situation and subsequent changes (red numbers). The first is the combustion of fossil fuels reserves, which produced 7.8 GtC/year in the decade 2000, taken as a reference for these data. The other numbers in red correspond to the system reactions that will be discussed in section 4.

The most important flow is related to photosynthesisBioenergetic process that allows plants, algae and some bacteria to synthesize organic matter from CO2 in the atmosphere using sunlight. Solar energy is used to oxidize water and reduce carbon dioxide in order to synthesize organic substances (carbohydrates). The oxidation of water leads to the formation of O2 oxygen found in the atmosphere. Photosynthesis is the basis of autotrophy, it is the result of the integrated functioning of the chloroplast within the cell and organism. of terrestrial vegetation, capturing about 100 GtC/year, theoretically able to extract all CO2 from the atmosphere in less than a decade. This is also known as primary production, which supplies organic matter to the entire earth’s life. This flow is almost exactly compensated by the CO2 emissions due to the breathingThis refers both to the gaseous exchanges resulting from the inhalation and exhalation of air by living organisms (CO2 carbon dioxide release and O2 oxygen absorption) and to cellular respiration which, by degrading glucose with oxygen, allows energy to be obtained., including that of the plants themselves and the bacteria that break down organic matter, as well as fires (e.g. forest fires); (read Peatlands and marshes, remarkable wetlands).

This primary production varies greatly in the ocean, regionally and seasonally, as phytoplankton are extremely sensitive to environmental fluctuations. Thus, the phosphate and nitrate richness of some waters (e.g. at the mouths of some rivers) locally fertilizes the surface layer of the ocean and leads to explosions in planktonic productivity (we speak of plankton blooms). This increase in photosynthesis reduces the CO2 content of surface water, which is compensated by a dissolution of atmospheric CO2. Phytoplankton and algae are grazed by consumer organisms (zooplankton, fish, molluscs) that regenerate most of the CO2 through their respiration and decomposition.
In addition, dead organisms or faecal particles carry organic carbon deep down where they feed living organisms (called heterotrophs) that remain dependent on surface photosynthesis. However, there is a notable exception near volcanic fumaroles where life is maintained by chemosynthesis, a reaction similar to photosynthesis where solar energy is replaced by the chemical energy of hydrogen sulphide (see Microbes in extreme environments and the focus on Black smokers’ ecosystems).
Only a small proportion of the marine organic carbon produced at the surface, about 0.2 GtC per year, accumulates in ocean sediments and slowly decomposes there without oxygen, forming hydrocarbons on a scale of millions of years. Coal is of terrestrial origin, its production began with the appearance of the first large trees during the Carboniferous era, 300 to 350 million years ago (see The biosphere, a major geological player).
Carbonated shells of marine organisms sediment and dissolve at great depth under pressure, releasing carbonate ions. This dissolution enriches deep waters with carbonates, such as sedimentation and recycling of organic particles (“biological pump”). In contrast, in shallow basins, these shells accumulate and slowly form limestone that is entrained in geological processes. Together with organic sediments, they are the only oceanic carbon sinks for the ocean-atmosphere system. In return, a CO2 flux of about 0.1 GtC/year is re-emitted by volcanoes into the atmosphere.
The dissolution (alteration) of the limestone supplies the ocean with carbonates for 0.1 Gt/year (this dissolution, as well as that of silicates, also results in atmospheric CO2 dissolved in rainwater for a total of 0.3 GtC/year). This carbon passes through rivers, joining that caused by soil erosion. The ocean receives 0.9 GtC/year from rivers, but this input is almost compensated by an estimated 0.7 GtC/year of ocean degassing in the pre-industrial situation.
These exchanges between atmosphere, rocks and living beings have played a considerable role in the ancient history of the Earth (see The biosphere, a major geological player), leading to significant variations in atmospheric composition and climate. However, these natural geological flows remain low: they are typically a thousand times lower than those of photosynthesis and nearly a hundred times lower than those of fossil fuel combustion. Over the past century, it is human activities that control the transformations of our environment.
4. The accumulation of CO2 over the last century

This accumulation of atmospheric CO2 induces global warming by increasing the greenhouse effect (radiation and climate link). It is also accompanied by ocean acidification that affects marine organisms.
The major contribution of fossil fuel combustion to the increase in atmospheric CO2 is confirmed by the isotopic composition of CO2 carbon (see Radioactivity and nuclear reactions). Carbon consists mainly of the isotope 12C (containing 6 neutrons in addition to the 6 protons), but also of a small proportion of 13C (7 neutrons) and 14C (8 neutrons). Photosynthesis captures 13C less efficiently than 12C so that biologically derived carbon has a lower isotopic ratio of 13C/12C than mineral carbon emitted by volcanoes. However, we observe that the isotopic ratio 13C/12C of atmospheric CO2 decreases, which marks its biological origin.
14C is produced by the nuclear reaction of cosmic radiation on nitrogen in the upper atmosphere, then integrated into CO2 and captured during photosynthesis, so that it is incorporated into all living beings in known proportions (10-12). As it is radioactive, its concentration decreases with a half-life of 5730 years (this is what is used for dating biological remains). It has therefore completely disappeared into much older fossil fuels.
However, there is a decrease in the atmospheric 14C concentration (after adjusting for the contributions of the nuclear explosions of the 1960s, which doubled the 14C concentration). This shows that the increase in CO2 comes from fossil biological sources [6] rather than from current biological sources.

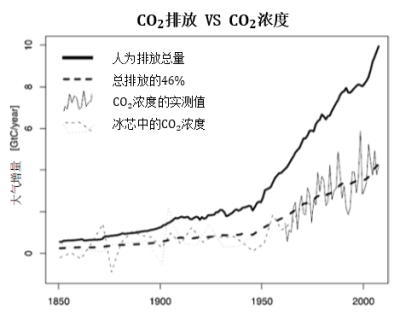
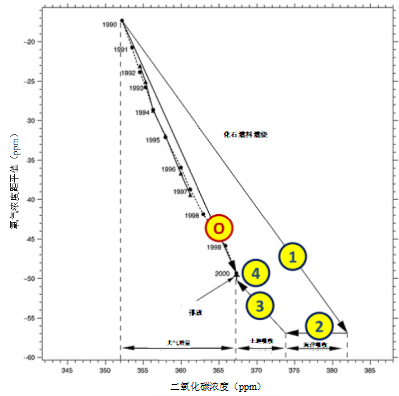
Over the decade 2000 shown in Figure 2, these figures translated into GtC (1 ppmv for 2.12 GtC, see section 2), increased to 7.8 GtC per year produced by fossil fuels, of which 2.3 GtC dissolved in the ocean and 1.5 GtC recycled by photosynthesis. The remainder, 4 GtC per year, has accumulated in the atmosphere.
In addition to CO2, carbon is also emitted into the atmosphere in the form of methane by anaerobic fermentation (away from the air) and by leaks from oil or gas fields. Although 200 times less concentrated than CO2, its contribution to the increase in the greenhouse effect is 25% of that of CO2 [4]. Its impact on the greenhouse effect is about 25 times more powerful than CO2 and its concentration in the atmosphere has increased by a factor of 2.5 compared to the pre-industrial situation. A sudden release of methane from frozen soils or marine sediments could accelerate global warming. However, the methane emitted into the atmosphere only persists for about ten years because it is slowly oxidized to CO2. Carbon is finally emitted in many other forms by human activities, in particular plastics produced at a rate of 0.3 Gt per year, of which an estimated one-tenth reaches the ocean and accumulates in the heart of vast eddies called gyres (see Plastic pollution at sea: the seventh continent).
5. Dissolution in the ocean
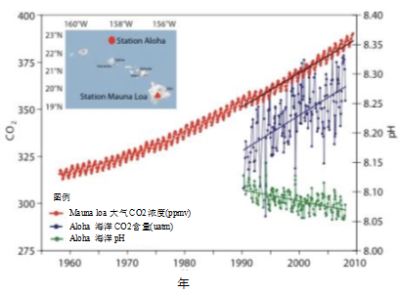
CO2 dissolves in the surface ocean, tending to reach a concentration of equilibrium proportional to its partial pressure in the atmosphere. This is a general property of gas solubility called Henry’s Law. This is why the dissolved CO2 escapes when you open a bottle of champagne, following the drop in pressure. This degassing is increased by an increase in temperature, which reduces solubility. CO2 thus escapes into the atmosphere in the warm tropical ocean, and on the contrary dissolves in the polar regions, the overall balance being neutral in a stationary regime. Global warming, on the other hand, induces global degassing. In the current period, however, it is the dissolution induced by the increase in atmospheric CO2 that dominates.
The surface ocean currently contains mineral carbon at 24 ppm by mass [9] (24 g per tonne of water). If we take the total mass of the ocean, 1.4 x 1018 tonnes, this would represent a reserve of 33 x 1018 g of carbon, or 33,000 GtC. The reserve is estimated at 38,000 GtC because the concentration is a little higher at depth. At equilibrium, the concentration of dissolved CO2 increases in proportion to its atmospheric pressure, in accordance with Henry’s law, but 95% of the dissolved carbon is in the form of carbonate and hydrogen carbonate ions. Thus the relative increase in dissolved carbon is only about 1/10 [10] of the relative increase in atmospheric CO2: an annual increase in this CO2 of 2 ppmv over 400 ppmv, or 0.5%, therefore implies a 10 times smaller increase in dissolved carbon, or 0.05% of 24 ppm, giving 0.012 ppm. This carbon is only absorbed by the surface ocean: if it were absorbed by the entire ocean, the flux would be 17 GtC per year, whereas it is estimated at 2.3 GtC/year (Figure 2). The absorbed CO2 is only transferred very slowly at depth.

In addition, the dissolution of CO2 releases H+ ions by reaction with water, which corresponds to an increase in acidity. This is measured by the pH, which is even smaller when the solution is acidic. This decrease is clearly visible in Figure 6. Acidification tends to limit the synthesis of the coral’s calcareous skeleton, in a variable way depending on the species [11].
However, this dissolution equilibrium is limited to the ocean in direct contact with the atmosphere, a layer of a few hundred metres thick stirred by the wind. The deep ocean remains unaffected by stratification: warmer and therefore less dense water floats on the surface. Surface water penetrates deep only sporadically and very locally into the polar regions where, after concentration of salt by evaporation, it can exceed the density of the bottom due to its cooling. It is estimated that 50 x 106 m3/s is the surface water flow that feeds the deep ocean, compensated of course by an equivalent global flow of deep water rising to the surface (the total mass of the ocean remaining unchanged). This represents 1.5 x 1015 tonnes per year, and the renewal of the deep ocean, 1.4 x 1018 tonnes, takes place in about 1000 years.
Thus, each year, 1.5 x 1015 tonnes of water in equilibrium with the current CO2 concentration (380 ppmv for the decade 2000) is replaced by deep water in equilibrium with the pre-industrial concentration of 280 ppmv, i.e. 30% lower. As we have seen, this represents a difference of 10 times less dissolved carbon, i.e. 3% or 0.7 ppm. Multiplying by the total water flow, we obtain a well of 1 GtC/year buried towards the deep ocean.
In addition, we have seen that the concentration of dissolved carbon increases by 0.012 ppm per year in the surface ocean, in a sufficiently stirred layer to homogenize over the decade. By choosing as an indication a thickness of 300 m for this mixed layer (note: this layer is thicker than the “surface ocean” in Figure 2 which is limited to the area of light penetration allowing photosynthesis) over the entire ocean surface, 0.36 x 1015m2, the mass of water concerned is 1.1 x 1017 tonnes, which leads to an annual stored carbon mass of 1.3 GtC/year in this mixed layer. Adding what is discharged to the deep ocean, 1 GtC/year, we find the 2.3 GtC/year deduced from atmospheric observations for the decade 2000. This global value of the absorbed flux is also confirmed by a mapping of the CO2 fluxes measured between the atmosphere and the ocean [8].
This flux must increase in proportion to the atmospheric CO2 concentration as long as warming does not affect ocean currents. However, this purely physical assessment is very schematic. It assumes constant flows of biological origin, linked to sedimentation and dissolution processes, which play a key role in the vertical mixing of dissolved carbon, with a flow of 11 GtC/year.
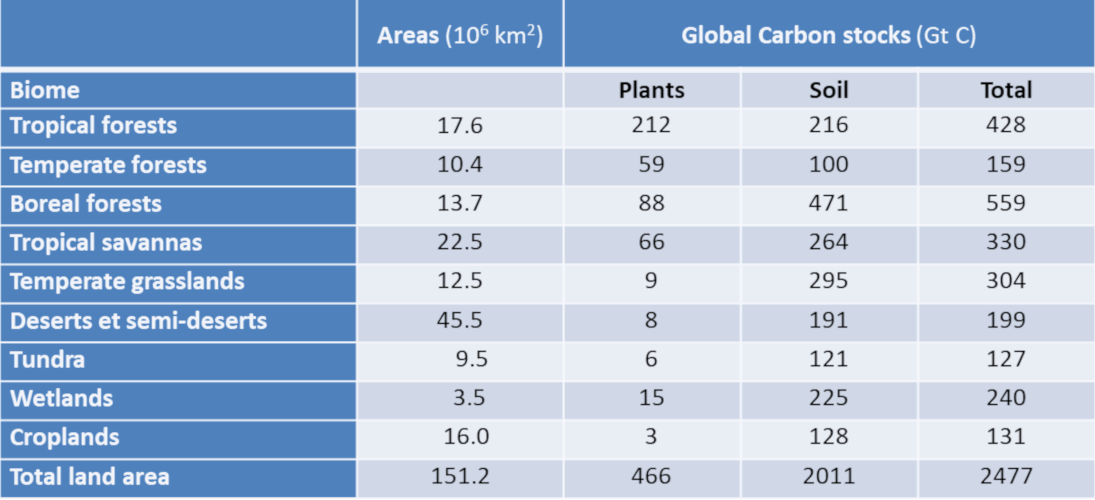
6. The influence of vegetation
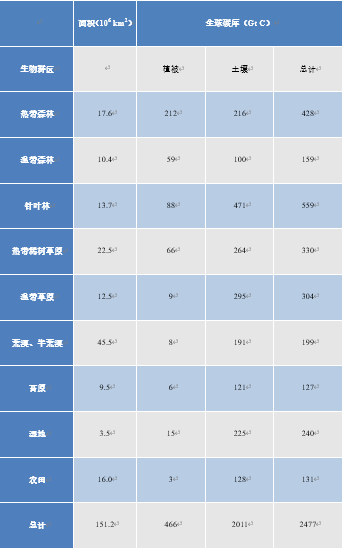
Terrestrial vegetation plays a key role in CO2 fluxes, as well as in the storage of carbon in the form of visible vegetation, but also in the form of humus in the soil. Thus grasslands, savannahs and even semi-desert regions contribute significantly to the overall stock, although their vegetation content is low, see Table 2 (see Peatlands and marshes, remarkable wetlands). It also shows that boreal forests store a large amount of carbon in the soil, while tropical forests have very abundant vegetation but soil that is not very capable of storing carbon.
Table 2. Overall carbon reserves in vegetation and upper (1 m) soil (Adapted from IPCC, 2001 [12]).
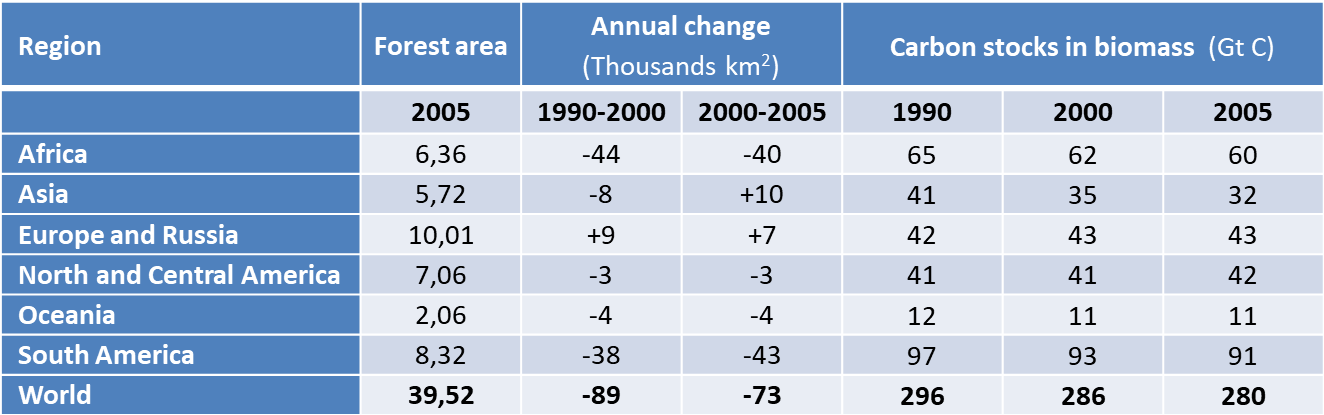
CO2 emissions from deforestation are now estimated at 1.1 GtC per year [13], contributing 25% to the rate of increase in greenhouse gases in the atmosphere. Deforestation has several origins: forest exploitation, the need for space for urban expansion, the conversion of forests into agricultural land, mining. For example, the boreal forest is destroyed over an area of 3,400 km2 by open-pit mining of the Athabasca oil sands (Alberta, Canada) [14]. Biofuel production by large scale agriculture is also devastating, promoting the development of monocultures, such as soya in the Amazon and palm oil in Indonesia, Malaysia and Thailand.
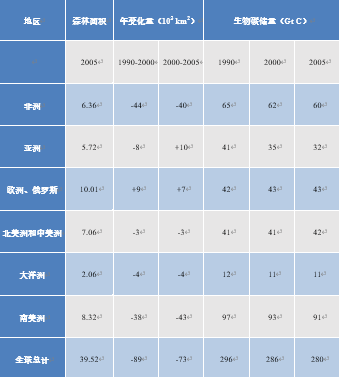
The rate of deforestation in tropical forests is of particular concern from both an ecological and a climatic point of view. It reaches 10,000 to 30,000 km2 per year in the Amazon [15], 5% of the forest area per decade in South America, with similar values in Africa (see Table 3). In Southeast Asia, fires encouraged by the El Nino climate phenomenon are helping to reduce forest cover. In 1997, forest and peatland fires in Indonesia released between 0.8 and 2.5 GtC of CO2 into the atmosphere [16]. Stabilization of forest areas in Asia includes massive destruction of primary forests and their replacement by planted forests, resulting in a reduction of nearly a quarter of the carbon stored by vegetation in 15 years (Table 3): from 12-74 kg/m2 in forests to 8.5-29 kg/m2 in oil palm plantations [17].
Table 3. Global deforestation in figures (Adapted from IPCC, 2007 [18]).
On the other hand, forests are spreading to other parts of the world, offsetting some of the deforestation. Inventories increased in Europe, North America and Oceania. In the mid-2000s, the overall net deforestation rate was about 70,000 km2 per year, slightly lower than the rate of about 90,000 km2 per year that prevailed [16] in the 1990s. This represents a relative loss of surface area of 2% per decade, and even more a loss of stored carbon of 3%, corresponding to 1.1 GtC per year (see Table 3).
However, the atmospheric balance of CO2 and oxygen (see Figure 5) indicates that photosynthesis absorbs 1.5 GtC per year overall. The 1.1 GtC carbon source due to forest destruction is therefore more than offset by a 2.6 GtC carbon sink per year. This “missing carbon” is probably captured in soils where the overall measurement is even more difficult than for vegetation. Several factors can lead to an increase in photosynthesis. It is known that increasing atmospheric CO2 concentration promotes photosynthesis if other environmental factors are favourable. The spread of nitrogen fertilizers and global warming could also encourage plant activity leading to increased storage of organic matter in the soil. The ocean could also contribute to this carbon sink, which would imply an increase in sedimentation from the estimate of 0.2 GtC per year shown in Figure 2, which corresponds to an average observed on older sediments.
7. The prospects for remediation of anthropogenic carbon emissions
As we have seen, the burning of fossil resources and deforestation lead to an accelerated accumulation of CO2 in the atmosphere, a source of global warming and ocean acidification. Only 50% of these flows are reabsorbed by natural feedbacks. A reduction of at least 50% in emissions is thus necessary to hopefully stabilize the CO2 concentration.
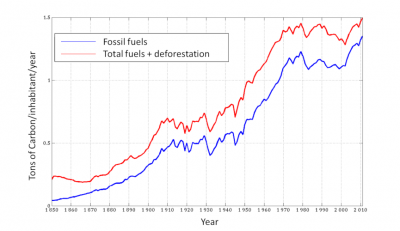
The per capita emission curve shows an inflection in the years 1970-2000 following the oil shock, the development of nuclear energy, and the decline of heavy industry (notably through the fall of the USSR). But there was a further increase in the 2000s following the massive use of coal, whose consumption increased by 50% in 10 years. It currently accounts for 30% of primary energy consumption (42% of CO2 emissions from fossil fuels) and oil 36% (28% of emissions). The contribution of decarbonated energies remains low (nuclear 6% of primary consumption, hydroelectricity 6%, other renewable 1%) [18]. Nuclear energy is reluctant among the population, but has the advantage of a minimal influence on the natural environment, unlike most renewable energies. These remain dominated by conventional hydropower. Wind and solar energy, which are still marginal, are developing rapidly. But they require the development of storage techniques, or additional fossil fuels, to compensate for the intermittency of their activity. In addition, their impact is limited to electricity production, which accounts for hardly a third of the primary energy consumed.
Methods for deep CO2 capture have been proposed, either in geological formations or on the ocean floor, by adapting mining and petroleum techniques. However, their long-term reliability is difficult to demonstrate and their energy consumption is considerable. Their development would thus only aggravate the problem of energy resources, leading to the exploitation of “unconventional” deposits that are increasingly devastating for the environment.
It is probably by limiting deforestation and making the best use of agricultural resources that the most effective gains can be achieved in the medium term. Agriculture occupies 1/3 of the ice-free land area (12% for crops, 22% for pastures), and is estimated to absorb ¼ land primary production [19] (total organic matter production). Livestock is a source of 15% of greenhouse gas emissions, more than transport. Biofuels currently on the market (known as first generation) compete with food crops and cause deforestation. However, research on second- and third-generation biofuels raises hopes of using agricultural waste, improving current methanisation techniques (methane production by air-free fermentation).
References and notes
[1] However, it is necessary to mention the exception of Carbon 14 (constituting a proportion 10-12 of the carbon of living beings). This carbon isotope is produced in the upper atmosphere by the impact of cosmic radiation on nitrogen, then is transformed back into nitrogen by radioactivity with a period of 5730 years.
[2] Chen et al (2014) Hidden carbon in Earth’s inner core revealed by shear softening in dense Fe7C3. PNAS 111, 17755-17758, http://www.pnas.org/content/111/50/17755.full.pdf
[3] To do this, we notice that the total atmosphere contains 10.3 t/m2: it is the mass of the air column whose weight produces the average atmospheric pressure 1.013 x 105 N/m2. The mole (NA molecules where NA is the Avogadro number) of CO2 containing 12 g of carbon and 32 g of oxygen, or 44 g, while an atmospheric mole contains 29 g (weighted average between nitrogen and oxygen), the mass proportion of CO2 is 44/29 = 1.52 times its proportion in molecules 400 x 10-6, or 607 x 10-6 of 10.3 t/m2, equal to 6.25 kg/m2 CO2. The mass proportion of carbon in CO2 being 12/44 = 27%, we find that 400 ppmv represents 1.69 kg/m2 of carbon or 860 GtC.
[4] IPCC Report 2013, chapter 6: Carbon and Other Biogeochemical Cycles, http://www.ipcc.ch/report/ar5/wg1http://www.climatechange2013.org/images/report/WG1AR5_ALL_FINAL.pdf
[5] Knorr W. (2009) Is the airborn fraction of anthropogenic CO2 emissions increasing? Geophys. Res. Letters 36, L21710, http://radioviceonline.com/wp-content/uploads/2009/11/knorr2009_co2_sequestration.pdf
[6] Levin I. & Hesshaimer V. (2000) Radiocarbon-a unique tracer of global carbon cycle dynamics, Radiocarbon 42, 69-80, http://archiv.ub.uni-heidelberg.de/volltextserver/6862/1/LevinRAD2000.pdf
[7] IPCC Report 2001: http://www.ipcc.ch/ipccreports/tar/wg1/108.htm
[8] Takahashi T. et al (2009) Climatological mean and decadal change in surface ocean pCO2, and net sea-air CO2 flux over the global oceans. Deep-Sea Res. II 56, 554-577. Results summarized on http://www.ldeo.columbia.edu/res/pi/CO2/carbondioxide/pages/air_sea_flux_2010.html.
[9] Feely R.A., Doney S.C. & Cooley S.R. (2009) Ocean acidification, special issue Oceanography 22 (4), http://tos.org/oceanography/issue/volume-22-issue-04.
[10] This increase factor, also dependent on temperature, is called the “Reveal Factor”, see Zeebe R. http://www.eoearth.org/view/article/154468
[11] Guinotte J.M. & Fabry V.J. (2008) Ocean acidification and its potential effects on marine ecosystems. Annals NY Acad. Sci. Volume 1134, The Year in Ecology and Conservation Biology, pp. 320-342.
[12] IPCC Report (2001), Working group 1, Chapter 3, The Carbon Cycle and Atmospheric Carbon Dioxide, https://www.ipcc.ch/ipccreports/tar/wg1/pdf/TAR-03.PDF; Biome: A set of ecosystems characteristic of a biogeographical area and named from the vegetation and animal species that predominate and are adapted to it.
[13] Houghton R.A. et al (2012) Carbon emissions from land use and land-cover change, Biogeosciences 9, 5125-5142, http://www.biogeosciences.net/9/5125/2012/bg-9-5125-2012.pdf
[14] Government of Alberta (2008) Alberta’s Oils Sands. Resourceful. Responsible. Government of Alberta, http://environment.gov.ab.ca/info/library/7925.pdf
[15] Laurance W.F. & Bierregaard R.O. (Eds.) (1997) Tropical Forest Remnants: Ecology, Management, and Conservation of Fragmented Communities. University of Chicago Press, Chicago, Illinois.
[16] Page S.E., Siegert F., Rieley J.O., Boehm H.-D.V., Jaya A. Limin S. (2002) The amount of carbon released from peat and forest fires in Indonesia during 1997, Nature 420, 61-65.
[17] Ziegler, A.D., Phelps, J., Yuen, J.Q., Webb, E.L., Lawrence, D., Fox, J.M., Bruun, T.B., Leisz, S.J., Ryan, C.M. & Dressler, W, (2012) Carbon outcomes of major land-cover transitions in SE Asia: great uncertainties and REDD+ policy implications. Global Change Biology 18, 3087-3099. http://onlinelibrary.wiley.com.gaelnomade.ujf-grenoble.fr/doi/10.1111/j.1365-2486.2012.02747.x/full
[18] IPCC Report (2007) 4th Report. Part 3, Mitigation, chapter 9, Forestry https://www.ipcc.ch/pdf/assessment-report/ar4/wg3/ar4-wg3-chapter9.pdf; table based on FAO data (2006), Global Forest Resources Assessment 2005. Progress towards sustainable forest management.FAO Forestry Paper 147, 320 pp.; MEA (2005) Millennium Ecosystem Assessment. Ecosystems and human well-being: Scenarios. Findings of the Scenarios Working Group. Island Press, Washington D.C.
[19] https://en.wikipedia.org/wiki/World_energy_consumption
[20] https://en.wikipedia.org/wiki/Primary_production
The Encyclopedia of the Environment by the Association des Encyclopédies de l'Environnement et de l'Énergie (www.a3e.fr), contractually linked to the University of Grenoble Alpes and Grenoble INP, and sponsored by the French Academy of Sciences.
To cite this article: JOYARD Jacques, SOMMERIA Joël (April 23, 2019), A carbon cycle disrupted by human activities, Encyclopedia of the Environment, Accessed November 23, 2024 [online ISSN 2555-0950] url : https://www.encyclopedie-environnement.org/en/life/carbon-cycle-disrupted-by-human-activities/.
The articles in the Encyclopedia of the Environment are made available under the terms of the Creative Commons BY-NC-SA license, which authorizes reproduction subject to: citing the source, not making commercial use of them, sharing identical initial conditions, reproducing at each reuse or distribution the mention of this Creative Commons BY-NC-SA license.