表观遗传学——环境对基因表达的影响

我们生活的环境会影响我们的基因组,这被称为“表观遗传修饰”。例如,拥有相同遗传基因的同卵双胞胎可能会因为各自的生活环境而发育出不同的外貌或体态。目前,越来越多的专家学者认为,人类基因以一种复杂的方式来感知环境,进而影响我们的健康和行为。饮食习惯、迭代性疾病、摄入的药物和毒素、焦虑和压力、生活方式和居住区域等都可能会改变宿主细胞和它们的DNA。因此,同卵双胞胎的生命轨迹也会出现巨大差异:一个可能日趋肥胖,另一个则没有;一个可能发育正常,而另一个则患有精神疾病。很多动物和人类的案例均表明了表观遗传学[1]在改变后天性状中的作用。
1. 甲基化:表观遗传学的基础
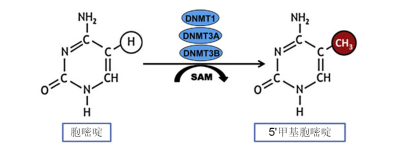
基因可以通过不改变DNA 序列的化学修饰(DNA甲基化或组蛋白修饰)来表达或抑制,DNA 缠绕在这些蛋白质上以形成染色质[2]的主要蛋白质,是DNA缠绕的线轴。这些化学修饰组成了“表观遗传标记”[3],归类于表观基因组(图1、图2)。广义上的表观遗传修饰是由环境诱发的:细胞不断接收各种外界信号,了解其所在环境,从而在生长过程中逐渐特化,或适应环境(见《表观遗传学:基因组及其环境》)。这些包括个人行为习惯(如饮食、吸烟、压力等)的信号会改变我们的基因表达,但不会改变我们的DNA 序列。表观遗传变化可能是暂时的,也可能是永久的。当诱导基因表达改变的信号消失时,这种改变会一直持续。与不可逆的基因突变不同,表观遗传标记是可以改变的。一个小小的环境变化就可以改变我们遗传基因的表达方式,进而改变我们的“表型”[4]。
表观遗传修饰具有自我调控因素,无论是远离基因的嵌入DNA序列的调节结构,还是可移动的小分子都会参与真核生物的诸多生化过程,如消除甲基化、组装染色质、抑制应激反应中蛋白质的生成等。这种可移动的小分子被称为微RNA,也被称为小分子RNA。微RNA比信使RNA小很多,它们可以破坏信使RNA,从而中断信息传递。
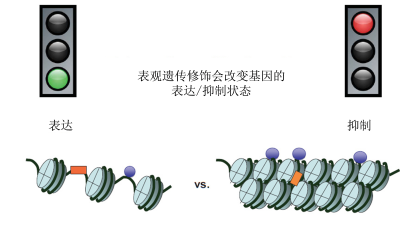
通俗来讲,如果说染色体是一盘磁带,而每个基因对应磁带上记录的每段轨迹,表观遗传修饰则是可以复位的胶带碎片,它可以掩盖某些轨迹,使其不可读,或展露某些轨迹,使其可读。表观遗传修饰常通过甲基化过程完成,它可以使DNA和组蛋白甲基化。乙酰基也有刺激基因表达的作用,可以使组蛋白乙酰化。
2. 表观遗传修饰对动物的影响
2.1. 概况
有些表观遗传标记可能会遗传给后代。研究表明,植物中通过DNA甲基化的表观遗传标记往往会代际传递。哺乳动物的表观遗传修饰能否遗传给后代是一个更加复杂的问题,仍然存在较大争议。本节中,我们通过刺鼠基因实验、果蝇红眼实验和老鼠交叉哺育实验等三个案例,来探究环境和个体历史是否会影响动物的基因表达。
2.2. 刺鼠基因实验

直到20 世纪末,科学家们才发现刺鼠,这让科学家们意识到食物和表观遗传学之间存在复杂的关联。在众多影响刺鼠皮毛颜色的基因中,有一种叫Agouti,也被称为“黄色皮毛表达基因”或“Avy”(图3)。如果Avy基因没有被甲基化,那么它在所有细胞中都被表达,刺鼠呈黄色。这些黄色刺鼠易患肥胖症、糖尿病或癌症。如果Avy基因高度甲基化,那么它在所有细胞中的表达都被抑制,刺鼠则呈棕色。虽然棕色刺鼠和黄色刺鼠拥有完全相同的基因,但棕色刺鼠都很健康。Avy基因也可以部分甲基化,这会影响基因的活性水平[5],导致有渐变斑点的刺鼠的出现。斑点刺鼠的Avy基因活性因细胞而异。正是由于这一在母体中就形成的表观遗传差异,一窝基因相同的刺鼠皮毛颜色会不尽相同。此外,无论皮毛颜色如何,饮食对甲基化也有一定影响。美国研究员兰迪·吉尔特尔有一个惊人的发现。他给一些携带Avy基因的刺鼠的食物中加入了维生素B。他虽然没有治愈那些患有遗传病的刺鼠,但这些刺鼠的后代十分健康[6]。也就是说,用维生素B喂养携带Avy基因的刺鼠,其后代不再患病,甚至不再呈现黄色皮毛(Avy基因仍然存在,但不再表达);而没有服用维生素B的刺鼠,其后代仍然患有遗传病。
2.3. 果蝇红眼实验

果蝇的基因组相对简单,因此它们是实验室里常见的昆虫之一。2009年4月,巴塞尔大学的雷纳托·帕罗博士公布了一项喜人的新发现:如果将孵化前的果蝇卵加热到37°,孵化后果蝇的眼睛将变为红色,反之则为白色。而且,红眼性状将代代相传。这说明,由外部因素(如温度)影响的性状会世代相传[7]。这些动物研究为以拉马克主义为代表的科学理论提供了实证依据。由他在1809年出版的名著《环境的影响》一书可知[8],个体在一生中所经历的身体变化是可以遗传给后代的[9]。然而,我们尚未证实人类的表观遗传修饰能持续多少代。因此,这些结果并没有真正推翻达尔文关于物种长期进化的观点,即物种会通过自然选择筛除掉偶然的遗传变异(见:《进化论:误解与抵制》和《适应:应对环境挑战》)。
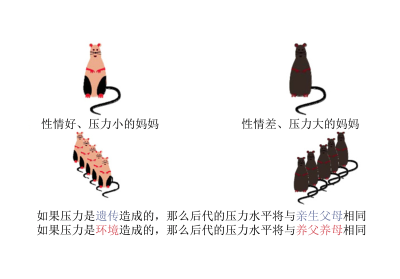
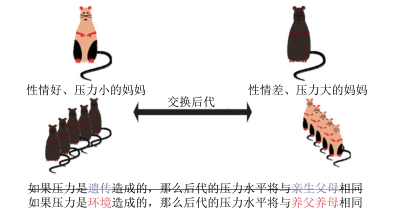
2.4. 老鼠交叉哺育实验
简单的爱抚会影响基因表达吗?老鼠之间的舔舐与人类之间的爱抚具有相同的作用。研究表明,经常被母亲舔舐的幼鼠性情更好。加拿大麦克吉尔大学的迈克尔·梅尼教授课题组在幼鼠大脑的海马体(应对环境压力的大脑区域)区域进一步揭示了母鼠舔舐对幼鼠大脑的影响[10]。
事实上,舔舐影响了老鼠NRC31基因的活性,该基因会产生一种蛋白质(即糖皮质激素受体),有助于降低体内应激激素(皮质醇)的浓度[11],从而使老鼠能够应对压力。然而,该基因的特定部分必须通过DNA甲基化来激活。对没有接受足够舔舐的小鼠脑部分析显示,与NRC31基因相关的开关(即糖皮质激素受体的基因编码)在小鼠海马体的神经元中存在缺陷(基因经过甲基化而失效)。因此,血液中应激激素(皮质醇)含量增加,即使没有干扰因素,他们也会处于持续的压力之中。
3. 食物对表观遗传修饰的影响

“荷兰大饥荒”事件证明了表观遗传学和人类饮食之间存在一定关联。1944年冬,由于德国纳粹的封锁,荷兰西部人民经受了饥荒[12],为科学家提供了一个真实的实验场所来验证表观遗传学和饮食之间的关系。
研究表明,经历饥荒的孕妇的孩子多患有糖尿病、肥胖症、心血管疾病、肾功能障碍(尿中白蛋白)等疾病。此外,他们的体型比正常人要小,他们生育的孩子也会比一般人体型小[13]。
数据表明,经历饥荒的母亲产生了表观遗传变化,并遗传给了她们的后代。这些孩子遗传的基因记录了这一事件,说明个体是否健康是由其胎儿发育阶段时所处的环境决定的。这些对体型的影响来自于DNA中表观遗传标记的改变,这与该时期荷兰祖先饮食中缺乏氨基酸和维生素等重要分子有关。为了在细胞分裂中保持正常甲基化水平,旧细胞必须向新复制的DNA中添加新的甲基。然而,这种甲基的持续供给来源于食物中的氨基酸和维生素(如蛋氨酸、甜菜碱或胆碱)。此外,我们还可以从化学前体(如叶酸)中制造甲基。
2008年,研究人员进一步阐释了这些表观遗传变化[14]:缺乏营养的个体在子宫内发育时,有较少的甲基团附着于控制生长因子IGF-2(胰岛素样生长因子-2)的基因上。甲基团的输送和DNA附着也需要食物中的其他化学元素(如锌、维生素B12等)。
4. 讨论和结论
虽然基因组是无法改变的,但表观基因组却各不相同。表观遗传修饰能让个体快速适应环境变化,而且不需要把这种变化“刻”进其基因组。表观遗传学不仅涉及医学和公共卫生学(见《表观遗传学:基因组及其环境》),还涉及进化学(见《进化论:误解和抵抗》)。事实上,表观遗传学关注的是那些可能会通过调节某些基因的活性来改变我们的性状,甚至诱发某些代际传染病的外部环境。显而易见,1944年冬的荷兰大饥荒导致当时孕妇的遗传基因发生了永久性改变,并世代相传。这意味着恶劣的环境也会影响生殖细胞(精子和卵子),因为这是两代人之间唯一的生物学联接。
现在人们普遍认为表观遗传异常有助于探究一些人类疾病,特别是癌症的发生和发展。在细胞分裂、细胞分化、细胞生存、细胞迁移等事件的调节中都涉及表观遗传过程。这些机制的交替发生使健康细胞转化为癌细胞,任何表观遗传异常都可能参与癌变。研究发现表观遗传异常会激活癌基因(致癌基因过度表达)或抑制抑癌基因。同理,在肿瘤细胞中也发现了影响编码表观遗传标记酶的基因突变。这些现象是癌症发病的原因还是结果尚待进一步研究,但表观遗传异常似乎参与了肿瘤的发展。
此外,表观遗传学在较复杂和多因素疾病中的作用得到了广泛研究,如神经退行性疾病(阿尔茨海默病、帕金森病、肌萎缩侧索硬化症、亨廷顿病等)以及代谢性疾病(肥胖症、2型糖尿病等)。正如我们知道如何获得一个完整的基因组序列,我们也可以知道表征该序列的所有表观遗传修饰,即表观基因组。这种整体、公正的方法会让人们更好地理解表观遗传学在人类疾病中的作用。
端粒酶
除了半世纪前由英国生物学家康拉德·沃丁顿(1905-1975)[15]提出的表观遗传过程外,2009年诺贝尔医学与生理学奖获得者伊丽莎白·布莱克本和卡罗尔·格雷德在分子生物学的重大发现也至关重要,同样强调了环境的影响。他们发现了一种被称为端粒酶的酶。这种酶可以调节“端粒”长度。端粒是指位于每个染色体末端非编码 DNA的重复片段(见《减缓衰老的焦点:端粒酶轨迹?》)。这些端粒类似于用于保护鞋带尖端的小塑料片或金属片。这些端粒在染色体末端形成保护帽,防止遗传物质损失。端粒也是衰老的标志,它们会随着时间的推移逐渐缩短。然而,端粒酶可以帮助端粒停止缩短甚至延长。因此,老化是一个动态过程,可以被加速或减缓的。伊丽莎白·布莱克本和营养学家、医学博士迪恩·奥尼斯的研究发现,改变生活方式能延长端粒(见《减缓衰老的焦点:端粒酶轨迹》)[16]。
尽管科学家们对拉马克“后天性状的可遗传性”这一主张重新燃起了兴趣(见《拉马克和达尔文:两种对待生命世界的不同看法》),环境在表观遗传学中的深远影响尚待进一步研究。学者们最关心的还是表观遗传过程在进化中的重要性。针对该论题,学界仍然存在分歧,主要问题在于:经过足够多的世代传递,表观遗传是否会产生自然选择?医生和社会学家则会关心另一个更为迫切的问题:我们的生活方式是否比遗传基因更重要?
参考资料及说明
封面图片:这一对双胞胎具有相同的基因组,但他们的表观基因组和表型却存在差异,因为他们生活在完全不同的环境中。[图片来源:Photo © Peter Voerman photography via Visual Hunt / CC BY-NC.]
[1] 表观遗传学:在没有DNA突变的情况下,通过细胞分裂或世代传递的基因活性过程。这种“基因活动”是基于染色质状态或“表观遗传标记”的。
[2] 染色质:是染色体的一种基本物质,由DNA分子组成,包裹在“组蛋白”周围。
[3] 表观遗传标记:染色质中DNA或相关蛋白质的化学修饰。这些修饰(例如胞嘧啶甲基化)有助于调控基因活性,但并不影响核苷酸序列,即在DNA上书写基因信息的“字母”(A、T、C或g)。
[4] Jablonka E & Lamb M, (2005), 四个维度的进化, 剑桥, 麻省理工学院出版社。
[5] Morgan HD et al., (1999), 刺鼠基因的表观遗传, 自然遗传学, 23:314-318。
[6] Jirtle, RL & Tyson, FL, eds., (2013), 健康和疾病中的环境表观基因组学:表观遗传学和复杂疾病起源, 海德堡:斯普林格。
[7] G & R Paro Shit, (2009), 关于肿瘤:表观遗传学与信号传导, 发育细胞, 17:440-442。
[8] Lamarck JB, (1809-1810), 法国巴黎自然历史博物馆, 《动物行动对环境的影响》。
[9] Charles Darwin (1809-1882)的进化论:物种的进化是随机发生的“变异”的结果,并且会遗传给后代,环境会选择更具有适应性的变异,这就是自然选择。Jean-Baptiste de Lamarck(1744-1829)所认可的进化:环境会为个体带来有益的变异,这种变异会代际传递,这种“后天性状的遗传”会促进物种进化。
[10] Francis D. et al., (1999), 大鼠中母性行为和应激的跨代非基因组传播, Science, 1155-1158.
[11] Weaver IC. et al., (2004), 通过母体行为进行表观遗传编程, 自然神经科学, 7:847-854.
[12] Lumey LH. et al., (2007), 荷兰冬季大饥荒家庭研究, 国际流行杂志, 36: 1196-1204.
[13] Stein AD. et al., (2004), 子宫内营养不足与出生时身体比例之间的关系:荷兰冬季大饥荒, 国际流行病学杂志, 33:831-836.
[14] Heijmans BT. et al., (2008), 胎儿阶段经历饥荒所导致的持续表观遗传差异, PNAS, 17046-17049.
[15] Baedke J, (2013), 随时间推移的表观遗传景观:康拉德·哈尔·沃丁顿对生命科学的方法论影响.历史和科学哲学研究: 生物及生物医学历史和哲学研究, 44:4, 756-773.
[16] Ornish D., (2008), 增加端粒酶活性和生活方式转变, Lancet, 2008, 9(11):1048-57.
环境百科全书由环境和能源百科全书协会出版 (www.a3e.fr),该协会与格勒诺布尔阿尔卑斯大学和格勒诺布尔INP有合同关系,并由法国科学院赞助。
引用这篇文章: DROUET Emmanuel (2024年3月9日), 表观遗传学——环境对基因表达的影响, 环境百科全书,咨询于 2025年4月8日 [在线ISSN 2555-0950]网址: https://www.encyclopedie-environnement.org/zh/sante-zh/epigenetics-how-the-environment-influences-our-genes/.
环境百科全书中的文章是根据知识共享BY-NC-SA许可条款提供的,该许可授权复制的条件是:引用来源,不作商业使用,共享相同的初始条件,并且在每次重复使用或分发时复制知识共享BY-NC-SA许可声明。









