汞、鱼和金矿工人

汞很能体现评估和管理环境健康风险的难度。在食物链中这种金属的存在已被证实具有潜在的风险,这必须与食用某些食物(比如鱼)的好处相权衡。汞及其健康效应的历史,通常以一些重大事件为标志,表明了几十年在环境健康方面取得的进步。
1. 汞

[资料来源: 昆汀·马西斯之后[公共领域], 通过维基共享资源.]
汞是一种银白色、有光泽、密度大、可流动的金属,因此长期以来也被称为水银。它的化学符号Hg来自其拉丁名“hydrargyrum”;水银中毒(hydrargyrism)这个词有时仍然用来形容汞中毒。“汞”一词出现在17世纪。早在1919年职业汞中毒就被录入法国职业病记录。在自然界中,汞与硫结合形成硫化汞,即“朱砂”;它在西班牙、阿尔及利亚和中国仍然以这种形式被开采;而在法国,使用的大部分汞来自废料再加工。
简单来讲,汞以不同形态存在:元素汞或金属汞、无机汞盐和有机汞[1]。长期以来,汞金属的特性使其在工业界取得了成功。汞的化学性质相对稳定,是唯一在室温下呈液态且易挥发的金属,可溶解许多金属形成汞合金(汞齐)[2]。
[资料来源: 巴塞洛缪·斯特伯(Bartholomew Steber) [公共领域], 通过维基共享资源.]
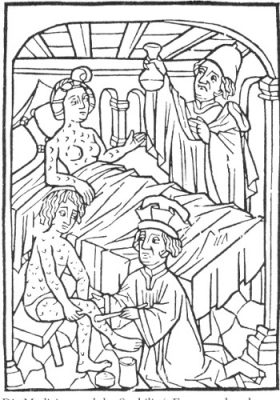
我们不能不把汞和中世纪著名医生和炼金术士——帕拉塞尔苏斯联系起来。自从他公开焚烧希波克拉底和盖伦的著作以来,他成为了那个时代的叛逆者(图1)。
他通过观察矿工的肺部病理开创了职业医学,是第一个将汞引入治疗(尤其是梅毒)的人(图2)。当他注意到汞的许多负面效应时,他说了一句至今在毒理学界很著名的话:“剂量决定毒性”。当时关于梅毒流行这样一种说法——“一夜维纳斯,千夜汞相随”!
1.1. 金属汞
金属汞对人体的毒性来自于其在室温下的高挥发性,即“蒸气”风险。这就是为什么1999年法国禁止在“大众”设备(如温度计、气压计等)中使用金属汞。这也是为什么高中生不再看到老师手里滚着几个水银球来上课(图3),年长的学生可能会对此记忆犹新。经口摄入后金属汞不会通过消化道屏障,因此不会被身体吸收。换句话说,这种形式的汞不再是公共健康问题,但本文仍然会讨论金属汞。

[资料来源: A [公共领域], 通过维基共享.]
[资料来源: 约翰·坦尼尔(John Tenniel) [公共领域], 通过维基共享.]
毒理学充满了关于金属汞的故事、轶事和参考文献。刘易斯·卡罗尔的《爱丽丝梦游仙境》便是一个例子。你可能知道这个故事中的疯帽匠(图4),也可能知道英语中“疯得像个帽匠”(mad as a hatter)这样的表达。事实上,刘易斯·卡罗尔只是密切观察了19世纪末英国工业时期发生在他周围的事。帽匠用加热的水银来制作和抛光帽子上的毛毡。吸入汞蒸气会导致行为问题。因此刘易斯·卡罗尔决定在这个著名的冒险故事中引入这个疯狂的角色。
1.2. 无机汞
除个别事故外,无机汞并不存在真正的公共健康问题。在一些实验室或纽扣电池中,仍然存在无机汞盐,但它对人体健康并没有严重的影响。然而,这并不妨碍人们积极寻找某些工业产品(如纽扣电池)的替代品。
1.3. 有机汞
对于普通人群,汞的问题主要就在于有机汞[3] [4]。人们通过饮食经常接触到的正是有机汞,而人类对此负有不可推卸的责任。1989年之前,人们将有机汞用作为杀真菌剂,来杀灭谷物中微小的真菌(后面我们将看到因此带来的严重后果)。有机汞会使中枢神经系统中毒,因此它在被暴露的个体中引发了严重的神经系统并发症。现在人们已经认识到了这个问题,法国公共卫生部建立了针对暴露人群的监督体系[5],并由法国国家食品环境及劳动卫生署定期发布健康建议[6]。
2. 有机汞,一个公共健康问题
有机汞的历史伴随着一些标志性的重大事件,同时也使我们更好地理解汞的毒性及其进入食物链的途径。
2.1. 水俣湾
一部真实的刑侦惊悚片发生在20世纪50年代的日本。故事从生活在水俣湾沿岸的渔民家庭出现严重的神经系统疾病开始。这是一起我们今天所说的“聚集性”病例,即病例都出现在同一区域内。
成年人有严重的瘫痪、神经精神障碍;即使不是死胎,许多儿童患有严重畸形,并伴有有严重的神经和心理障碍。尽管专家给出了许多意见,这些真实案例仍持续了很多年,这个谜团一直没有解开!最终有600人死亡,患病人数达3000人。当时,流行病学还是一门新兴科学,对生物体液(血液、尿液等)的毒理学分析仍然相当不成熟。我们可以想象日本的这个地区在一些年里一直受到一种神秘疾病的困扰,但病因却无人知晓。
但一些线索也渐渐显露出来,其中有两个特别的地方:一是这些家庭主要以海产品为食,二是当地一家大型化学公司——窒素公司,使用汞作为某些化学合成的催化剂,并定期将废水排放到这片太平洋海湾的水域。
随着故事的发展,来自苏格兰的毒理学家最终发现了事情的真相。他们是怎么发现的呢?首先,他们查看了所有病人的病历,寻找可能的共同点。他们得出的结论是:所描述的病理与当时已知的有机汞的毒性相似。然而,窒素公司的工厂向太平洋大量排放的是金属汞。如我们之前所了解的,只有汞金属蒸气对人体有毒,且摄入后的汞不会通过消化道屏障。这个谜持续了一段时间。
专家们最终还是怀疑窒素公司的工厂与这场“流行病”和渔民的病状有关系。
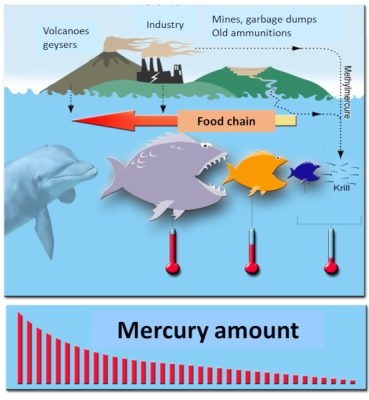
[资料来源: 拉米奥(Lamiot) (CC BY-SA 3.0), 通过维基共享资源.]
(译者注:volcanoes geysers 火山间歇泉;industry 工业;mines, garbage dumps 矿山、垃圾场;old ammunitions 废旧的军火弹药;methylmercure 甲基汞;food chain 食物链;krill 磷
虾;mercury amount 汞量)
随后人们发现了有机汞进入食物链的关键一步:存在于水中的金属汞被海洋微生物“甲基化”后变成了甲基汞!也就是说,工厂排放的金属汞被加上了一个甲基自由基[7]。这一被称为“生物甲基化”的现象随后备受关注。金属汞变成了有机汞,一旦被摄入体内,就会被迅速且充分吸收!
另一个如今在环境健康领域广为人知的现象是生物累积。汞是一种具有“累积性”的有毒物质,它会随时间推移在生物体内积累:鱼或哺乳动物的年龄越大,其体内累积的有机汞含量越高。人们还发现了另一种现象——生物放大,即大鱼吃小鱼。由于人类处在食物链顶端,有机汞通过食物链最后在人体内积累。所以,当人类食用旗鱼、金枪鱼和大比目鱼时会获取最大剂量的有机汞;食用其它大型鱼类同样如此(图5)。

[资料来源: Quirkyjazz via Flickr (CreativeCommons BY-NC-ND 2.0)
直到半个世纪后的2010年,这一重大事件的幸存者(大多患有严重残疾)才最终得到窒素公司和日本政府的赔偿。水俣市建造了许多代表金属汞球的雕塑,以示纪念(图6)。
2.2. 渔民和冰芯
被水俣湾事件所启发,一些科学家不禁要问自己这样一个问题:这是一起孤立的工业事故吗?还是自19世纪末工业革命以来,海洋已经被汞污染了?首先,值得注意的是,哺乳动物体内的汞会被人发和指甲及鸟类的羽毛等部分清除。正如自克劳德·伯纳德以来,科学的推进往往需要实验验证,科学家们提出了一个假设:海洋没有被污染,水俣湾事件是一起孤立的工业事故。

[资料来源: 美国宇航局[公共领域],通过维基共享资源(左图);卢西亚诺·吉萨尼(Luciano
Giussani) (CC BY 2.0),通过维基共享资源(右图)]
但是如何证明呢?科学家们想出了一个绝妙的主意:他们分析了自然历史博物馆中鸟类标本的羽毛,尤其是捕鱼鸟类,如鱼鹰和凤头鸊鷉(图7)。对鸟类羽毛的分析可以回溯到19世纪中叶。坏消息是,自工业时代开始以来,海洋的汞污染从未停止增长。最近的一项研究证实了这些结果[8]:在海鸟羽毛中测得的汞平均浓度为几μg/g。如果继续发展下去,汞浓度将在几十年后达到约20 μg/g的危险水平。
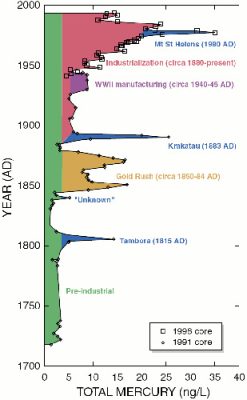
[资料来源:美国政府[公共领域],通过维基共享资源]
(译者注:Year (AD) 年(公元);Total Mercury (ng/L) 总汞量(ng/L);Mt St Helens (1980 AD) 圣海伦斯火山(1980 年);industrialization (circa 1880-present) 工业化(约1880年至今);WWII manufacturing(circa 1940-45AD) 第二次世界大战制造业(约1940-1945 年);Krakatau (1883 AD) 喀拉喀托火山(1883 年);Gold Rush(circa 1850-84 AD) 淘金热(约1850-1884 年);”unknown” “未知”;Tambora (1850 AD) 坦博拉火山(1850 年);Pre-industrial 工业化前)
另一些研究则集中在由空气污染引起的汞沉积上,特别是在极地冰川中的汞沉积。一项研究调查了一座位于中纬度美国怀俄明州的冰川[9]。这项针对冰芯的研究基本证实了人类对环境中大规模汞污染负有责任。图8 中绿色代表自然的“背景噪音”(自然沉积),蓝色代表该州的火山活动,而金色代表人类活动(如淘金者),红色则代表工业活动。这项研究显示,在过去100年里,70%的汞排放是由人类活动造成的。由于采取了多种减少汞排放的手段,汞浓度在过去15-20年里有所下降。
最近,有证据表明永冻层中(北半球的冻土)含有大量的汞[10];全球变暖可能会使这类汞逐渐释放,这会引起人们的担忧。
2.3. 伊拉克的饥荒
20 世纪70年代,伊拉克农村出现了一场可怕的饥荒。为了帮助这些人民,国家和多个协会(尚未被称为非政府组织) 分发了大量袋装谷物。很快,这些农民及其家庭成员出现了各种神经系统疾病。当时甲基汞是被用作谷物的杀真菌剂。事实上,谷物经甲基汞处理后是被用作种子,而不能食用。然而,袋子上的标签是用英语标注的,当地居民对英语一无所知。因此,人们在食用了这些经过处理的谷物之后,出现了中毒症状。幸运的是,在水俣病出现约20年后,人们很快便发现了甲基汞与这些症状之间的关系。然而,最终结果还是令人震惊,近650人死亡,6500人患病[11]。
悲剧的发生使得科学不断进步。汞的剂量-效应关系得到了更好的研究,从那时起,毛发毒理学成为研究慢性重金属中毒的一个标准。
2.4. 法罗群岛和塞舌尔
为增进我们对有机汞人类暴露及其毒性的认识,大量针对暴露人群及风险评估的研究正在进行当中。例如,在印度洋海域,自1985年设立了一个针对法罗群岛和塞舌尔儿童群体的监测项目[12]。这些儿童食用大量鱼类,是潜在的长期接触低剂量甲基汞的“良好见证者”。这些研究取得了一定进展,包括使用精细的认知测试来检测最微小的毒性迹象,或测定出生时脐带血中的汞含量。
然而,需要注意的是,这些被称为队列研究的流行病学研究极其困难。在整个研究过程中,可能会出现许多偏差。例如,鱼类可能含有其他污染物,或者被监测对象的生活中可能存在其他环境因素。因此,我们很难将所有的观测结果仅归因于汞。在所有关于环境对人类健康影响的针对性研究中,这是一个众所周知的普遍事实。
3. 牙科汞合金
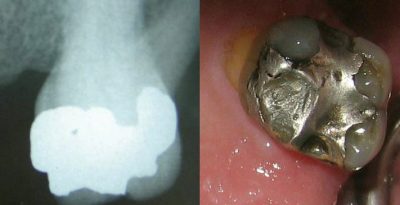
[资料来源: 德罗森巴赫(DRosenbach) [公共领域],通过维基共享资源]
牙科汞合金是一个敏感话题,我们很难平心静气地讨论它。由于金属汞可以与其他金属混合形成汞合金,它长期以来被用作龋齿的“填充物”(fillings)(图9)。顺便说一句,“fillings”的叫法是一种误称,因为它们不含铅,而含有银、锌、锡和铜(注:fillings 原特指之前流行的铅填充物)。
这些合金中所含的汞被认为是各种慢性疾病的罪魁祸首,如阿尔茨海默病或多发性硬化症。汞蒸气可以被吸入,从而进入体内。嚼口香糖和磨牙症[13]增加了汞蒸气释放的风险。迄今为止,尚没有任何国内外专家明确口腔中的合金与这些疾病之间的关系。
但是我们一谈论到牙科汞合金,还是会心潮澎湃!然而,后果也是我们不愿看到的: 欧洲国家的卫生从业人员声称可以以高价施用汞螯合产品来治愈患者。下面我们将看到,管理这种未经证实的风险非常困难。
4. 汞和淘金者

[资料来源: Maurizio Alì (CC BY-SA 4.0),维基共享资源]
水俣湾事件的“重演”发生在法国最大的省份圭亚那。秘密淘金者大量使用金属汞来混合可能存在于河水中的金块,然后将汞合金加热以回收黄金,每年都有一些人因吸入汞蒸气而死亡。
但更严重的是,这些淘金行为使这一大片美洲印第安人地区的所有河流都受到金属汞的严重污染(图10)。来自淘金者的金属汞被水体微生物转化为甲基汞,这使得包括法属圭亚那、巴西和苏里南部分地区在内的所有人口都暴露于甲基汞。负责人口监测的卫生机构——法国卫生组织早就制定了人口监测措施,并定期与法国国家食品环境及劳动卫生署沟通过度食用鱼类的风险(见参考文献[1]和[2])。
5. 汞和风险管理
汞很能体现管理环境健康风险的难度。风险管理在风险评估之后进行。
风险评估由独立科学家进行:他们根据目前的知识状况提出建议,并指出接触某种特定有毒物质的健康风险;通常他们还会建议不应超过的暴露限值。
风险管理是决策者的责任:法国的卫生机构(法国国家食品环境及劳动卫生署)及相关政府部门的部长就扮演了这一角色。这些决策者可能依据也可能不依据专家们的结论。我们举两个例子。
- 对于吃鱼有哪些建议?
除了汞、多氯联苯和二恶英等污染物之外,鱼类还会受到许多其他污染物的影响,这使问题更复杂了。风险管理总是要在“必须吃鱼”和“小心鱼里的有毒物质”之间寻求平衡。我们知道鱼类富含某些对健康非常宝贵的营养物质,比如富含脂肪的鱼类中的不饱和脂肪酸欧米伽3。我们还知道鱼类中的主要污染物及其健康风险(多氯联苯、二恶英、包括汞在内的重金属)。这些建议只是“保护性的”,还不能证明其有效性。因此,法国国家食品环境及劳动卫生署仅限制儿童和孕妇食用鱼类[14]。
- 关于牙科汞合金的建议?
这又带来了另一个问题,即在牙科手术中最常被用作汞合金替代品的树脂。但树脂也不是绝对安全的。根据法国国家药品和保健品安全署的预防原则,尽管最近所有的研究都没有证明汞的存在有风险,但有关部门对此的建议仍然是“保护性的”:避免幼儿和孕妇使用汞合金[15]。另一方面,牙科诊所中汞的使用多年来一直受到非常严格的监管,特别是为了避免牙科医生反复吸入及患者偶尔吸入有毒物质的风险。法国国家药品和保健品安全署[18]对患者[16]和专业人士[[17]均提出了建议。
几个世纪以来,对汞以及铅、砷或镉等大量重金属的不当使用造成了地球大规模、持续性的污染。不幸的是,这些金属都已被证明对人类健康有害。
汞对于大众来说是一个真正的公共健康问题,尤其是以有机形式存在的甲基汞。正如我们已经看到的,它进入了食物链,如我们吃的海鲜,尤其是鱼。但我们也要记住,我们必须吃鱼,但不能吃太多,也不能每天都吃。正如这种情况,当需要权衡利弊时,风险管理是一项困难的工作。
即使摄入金属汞不会造成任何危险,它也不应过度使用。海洋环境中的生物甲基化现象清楚表明,金属汞的环境污染是有机汞进入食物链的原因。
牙科汞合金也很好地说明了管理风险的难度。哪种风险存在?哪种不存在?无论对错,我们主要还是要遵循预防原则(详见专题预防原则)。
联合国环境规划署最近实施了一项关于汞的国际公约,即《水俣公约》。该公约2013年在日本通过,2017年8月16日生效[19]。在其主要条款中,《水俣公约》特别规定禁止开采新的汞矿,逐步淘汰现有汞矿,消除和逐步淘汰汞在一些产品和工艺中的使用,制定措施控制汞向大气、水和陆地的排放,并控制非正规手工和小规模金矿开采。《水俣公约》还涉及汞的临时储存及其成为废物后的处置、污染场地和健康方面的问题。
一段有趣的视频展示了全球汞污染的问题并正确看待该公约[20]:
6. 结果
- 汞很能体现管理环境健康风险的难度,它涉及到两步:
- 风险评估是由独立的科学家进行的。他们的建议是基于目前的知识状况,提出接触某种特定有毒物质的健康风险,以及不应超过的暴露限值。
- 风险管理是决策者的责任,相关的健康机构就扮演了这一角色。这些决策者可能会也可能不依据专家的结论来给出建议。
- 金属汞对人体的毒性来自于其在室温下的高挥发性。早在1919年法国职业病登记册上就记录了职业性汞中毒。
- 汞是一个真正的公共健康问题,尤其是以有机形式存在的甲基汞。
- 海洋环境中的生物甲基化现象表明金属汞的环境污染是有机汞进入食物链的原因。
- 汞是一种具有“累积性”的有毒物质,随着时间推移会在生物体内累积,这就是生物累积。
- 生物放大是环境健康方面的另一个重要问题:有机汞通过食物链积累,最后进入人体。人类处于食物链的顶端,当我们食用剑鱼、金枪鱼或比目鱼等鱼类时,会接触到最大剂量的有机汞。
- 风险管理总是在“吃鱼有益健康”和“小心鱼中的有毒物质”之间寻求平衡。
- 金属汞与其他金属混合形成汞合金的特性使其一直被用来作为龋齿“填充物”。
- 迄今为止,还没有专业知识能够证明牙科汞合金可能带来任何健康风险。
- 根据国家药品和保健品安全机构的预防性原则,他们给出的很多建议是保护性的。
- 多年来,牙科诊所对汞的使用一直受到非常严格的监管。
- 秘密采金者利用汞回收河水中可能存在的任何黄金。因此,这些河流(如法属圭亚那)被汞严重污染,造成了不可挽回的环境损害。此外,过度食用鱼类的人群也存在健康风险。
- 几个世纪以来,对汞以及铅、砷或镉等大量重金属的不当使用对地球造成了大规模、持续性的污染。不幸的是,这些金属都已被证明对人体健康有害。
参考资料及说明
封面照片:[资料来源:By Arnaud 25 [CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0)],来自维基共享]
[1] Bensefa-Colas L, Andujar P, Descatha A (2011). Intoxication au mercure. La revue de Médecine Interne, 32 (7), pp.416-24.
[2] 多种不同元素的组合。这个词在化学中最常用来指汞与其他金属的合金。
[3] 在《环境健康》中,明确区分了在工作场所面临某些风险的专业人员群体和普通人群(即工作场所以外的所有人)。
[4] Clarkson TW (2002). The three modern faces of mercury. Environment Health Perspectives, 110 Suppl 1: 11-23.
[5] 法国公共卫生 : www.santepubliquefrance.fr
[6] YEARS.国家食品、环境和劳动卫生安全局: www.anses.fr/fr
[7] Harada M (1995) Minamata Disease: Methylmercury poisoning in Japan caused by environmental pollution, Critical Reviews in Toxicology, 25:1, 1-24
[8] Bond AL, Hobson KA, Branfireun BA. 2015 Rapidly increasing methyl mercury in endangered ivory gull (Pagophila eburnea) feathers over a 130 year record. Procedings of the Royal Society of Biology, 282: 2015
[9] Schuster PF, Krabbenhoft DP, Naftz DL. 2001. Atmospheric mercury deposition during the last 270 years: a glacial ice core record of natural and anthropogenic sources. Environmental Science and Technology, 36, 2303-2310
[10] Shuster PF et al (2018). Permafrost stores a globally significant amount of mercury. Geophysical Research Letters, 45, 1-9
[11] Al-Tikriti K, Al-Mufti AW (1976). An outbreak of organomercury poisoning among Iraqi farmers. World Health Organization Bulletin. 53 Suppl:15-21
[12] Myers GJ, Davidson PW, Cox C and others (1995). Summary of the Seychelles child development study on the relationship of fetal methylmercury exposure to neurodevelopment. Neurotoxicology 16, 711-716.Encyclopédie de l’environnement 12/12 Généré le 13/08/2021
[13] 反复和无意识的牙齿摩擦
[14] YEARS:吃鱼的好处和风险.www.anses.fr/fr/content/manger-du-poisson-bénéfices-et-risques
[15] ANSM :牙科汞合金中的汞, 数据更新, 2015年4月.
http://ansm.sante.fr/content/download/76933/976017/version/1/file/ANSM_Report_Mercury-Amalgames-Dentaires_May-2015.pdf
[16] ANSM:http://ansm.sante.fr/var/ansm_site/storage/original/application/0b5892a526480ba401d0ccc3fd4ee0e2.pdf
[17] http://ansm.sante.fr/var/ansm_site/storage/original/original/application/7cacb0593aaa9f8ebd9b176c65ff98890.pdf
[18] ANSM :法国国家药品和保健品安全署: http://ansm.sante.fr/
[19] 关于汞的水俣公约:
http://www.mercuryconvention.org/Portals/11/documents/Booklets/COP1%20version/Minamata-Convention-booklet-fr-full.pdf
[20] 创作汞的历史: https://youtu.be/guO7RbArWf4
环境百科全书由环境和能源百科全书协会出版 (www.a3e.fr),该协会与格勒诺布尔阿尔卑斯大学和格勒诺布尔INP有合同关系,并由法国科学院赞助。
引用这篇文章: DANEL Vincent (2025年1月15日), 汞、鱼和金矿工人, 环境百科全书,咨询于 2025年1月22日 [在线ISSN 2555-0950]网址: https://www.encyclopedie-environnement.org/zh/sante-zh/mercury-fish-gold-miners/.
环境百科全书中的文章是根据知识共享BY-NC-SA许可条款提供的,该许可授权复制的条件是:引用来源,不作商业使用,共享相同的初始条件,并且在每次重复使用或分发时复制知识共享BY-NC-SA许可声明。







