“The Philly Killer”: Emergence of Legionella pneumophila, a ubiquitous environmental bacterium
PDF
July 1976, Bellevue Stratford Hotel in Philadelphia (Pennsylvania – USA, see Figure 1). The heat is stifling, the air conditioning is running at full speed. Several hundred veterans of the American Legion have gathered there for a convention. Following the convention, a sudden epidemic of severe pneumonia developed over several consecutive days in surrounding cities: 147 legionnaires were hospitalized, 29 (16%) died. No classical etiology was found. A genuine police investigation was launched under the aegis of the Centers for Disease Control and Prevention (CDCP) in Atlanta, to detect the “killer”: it remained unknown for several months. In fact, it was a new bacterium pathogenic to humans, later called “Legionella pneumophila”, whose biotope was fresh water. A new environmental disease was born: legionellosis!
1. 50 years ago: the emergence of legionellosis

From the onset of the epidemic, a team of approximately fifty epidemiologists from the Epidemic Intelligence Service (EIS) were deployed in the State, supported by numerous microbiologists, chemists and toxicologists (Figure 2). However, all searches for known causing agents remained inconclusive. Six months later, McDade reverting to Robert Koch’s postulates, inoculated a biopsy of lung tissue from a dead patient into a guinea pig and then into subcultures on embryonic eggs, which finally allowed the bacterial forms to be observed! During the following year, the main characteristics of the disease and the triggering infectious agent were defined: “Pneumonia caused by a Gram-negative bacillus, of difficult culture, occurring most often in ‘at-risk’ subjects (elderly men, smokers), mortality rate of 10%, entry through the respiratory tract, probable cause of transmission: air conditioning systems.” The epidemic was reported by the press worldwide and was even immortalized by a Bob Dylan’s song “Legionnaires’ disease“!

2. Identity card of “the killer bacteria” Legionella pneumophila
2.1. What is it?
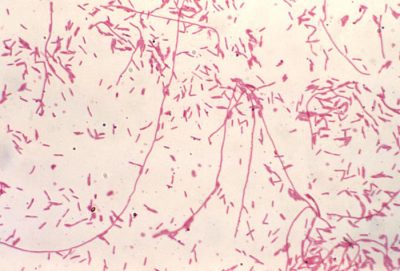
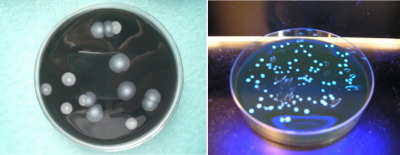
Initially, the bacterium is unable to multiply on existing media. In 1979, a new specific environment was described that is still relevant today. On average the division time of the bacterium is 4 hours; it explains, on the one hand, the time it takes for colonies to appear in cultures, between 2 to 5 days (Figure 4), and on the other, the incubation time of the disease, between 4 to 10 days. Several procedures for identifying colonies are possible: biochemical techniques, which were quickly abandoned, are now routinely replaced by immunological agglutination tests or mass spectrometry (MALDI-TOF-MS [4],[6]).
2.2. What are its biotopes?

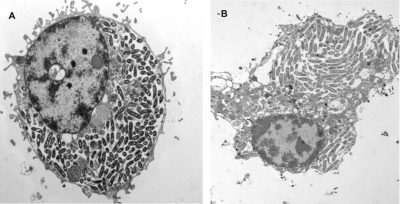
- In free living amoebae: Non-parasitic protists [7] complete their life cycle entirely in the environment and in water feed on microorganisms, including Legionella, by phagocytosis. They also have a natural “resistance” to amoebas; they internalize themselves in a vacuole called Legionella-Containing Vacuole (LCV) which constitutes a privileged breeding site in the environment ; thereby allowing Legionella to “shelter” in amoebas and resist environmental stresses (Figure 6) [11]. These eukaryotes (genera Acanthamoeba, Hartmanella, etc.) now become a potential reservoir of contamination.
- In bacterial biofilms [8],[12]: A biofilm (see Bacterial Biofilms and Health) is composed fromOF a dynamic environment where combined microorganisms enclosed in an extracellular matrix adhere to physiochemical surfaces: this model applies to Legionella. But biofilms can also be found on the surface of stagnant liquid media or “floating biofilms”: these play a crucial role in the persistence of bacteria in tanks which may be inaccessible and the bacterial biofilms are therefore difficult to eradicate with disinfectant products.

- In the form of viable but non-cultivable bacteria (VBNC) [13]: These appear under the effects of stress, which means that they are unable to multiply temporaly because of their low metabolic activity. However, if conditions become favourable again, they can regain their pathogenicity.
Artificial or man-made water towers: air cooling towers (Figure 7) and domestic hot water (DHW) installations [14],[15]. From natural sites, Legionella can colonize artificial sites and multiply if the temperature allows it to do so (25 to 45°C). Thus, any device or network containing hot water will constitute a potential niche for Legionella. Historically, air conditioning systems, cooling towers and evaporative condensers have been incriminated: hence, the word “air conditioning” is often synonymous with the word “legionellosis”! It has subsequently been proven that all “modern” domestic hot water networks could potentially be colonized with Legionella, and in zones more at risk, such as showers, whirlpools, decorative fountains, etc.
- Air-cooling towers installations were the first to be directly linked to the outbreak. Industrial hot water circuits or refrigeration units used in air conditioning (industry, trade) are favorable to the development of Legionella (water temperature and air/water contact in the installations). Humid air-cooling towers located outside buildings are cooling systems for these hot circuits, resulting in the formation of aerosols consisting of fine droplets which are released from the vents into the air. The extent of the risk will be greater or lesser depending on the location and density of the surrounding populations. Examples abound, a bus stop for golfers situated underneath the fallout, the involuntary recovery of vapors released by the buildings themselves or by neighboring buildings. The risks involving industrial cooling towers are less numerous. Albeit, the outbreak in Lens (1997) identified a cooling tower located 8 km, or even 16 km from the homes of contaminated patients (Figure 8)!

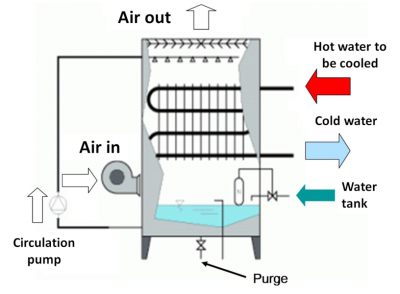
- Domestic hot water (Figures 9 & 10) is initially cold water destined for domestic use, and intended for human consumption and which will be heated secondarily. This was the second area of contamination discovered after Susan Fisher-Hoch’s observation of cases in England where the use of air conditioners is not very common [16]. The water distributed to households through the supply network is heated by various means such as boilers, hot water tanks, etc., which are either individual or collective. It is then distributed through an average sized pipe to any water outlet (taps, showers) where it becomes domestic hot water.
Legionella, potentially present in cold water in very low concentrations, will be able to multiply rapidly if the temperature conditions permit. Contaminated hot water becomes dangerous when it is transformed into aerosols: this is the case for example, for showers, spas, atomizers and mist devices, decorative fountains, or even in car washing installations and water jets used in dentistry.

In hospitals, the risks of contamination are identical but greatly increase with the admission of patients whose immunity has already been compromised (immunosuppression) or by their treatment (corticosteroid therapy). The epidemic of legionellosis which occurred in 2001 during the inauguration of the Georges Pompidou European Hospital remains a textbook case, underlining the importance of the composition of pipes as well as the time period of water stagnation in these pipes.
Other potential sites, although rarer, include wet soil such as “potting soil”, where compost can be contaminated by a particular species, L. longbeachae, which is also pathogenic to humans.
2.3. How did the bacteria emerge in humans?
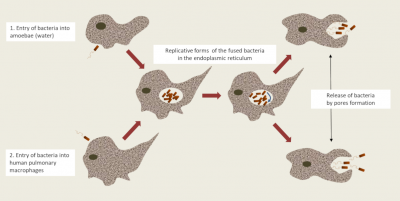
These particularities suggest that legionellosis is not a human communicable disease (only one exception was recorded in Portugal in 2016), but an opportunistic disease, the occurrence of which depends very much on many individual risk factors!
2.4. What is legionellosis?
- age over 50 years (legionellosis is rare in children and in young adults)
- smokers (45% of cases)
- males (75% of cases).
Other patients with a weakened immune system (malignant hemopathies, cancers, immunosuppressive treatments, prolonged corticosteroid therapy), chronic cardiac or pulmonary pathologies and diabetes will need to have highly regulated and supervised prevention and monitoring measures in place, including a mandatory “Zero Legionella” threshold for domestic hot water (DHW).
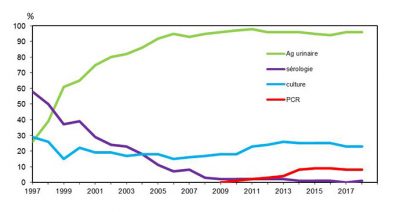
Legionellosis is a serious disease, hence the importance of an accurate diagnosis for therapeutic purposes but also for surveillance purposes. It is based on an association with pneumonia and the presence of at least one of the following 4 microbiological criteria [19] (Figure 12):
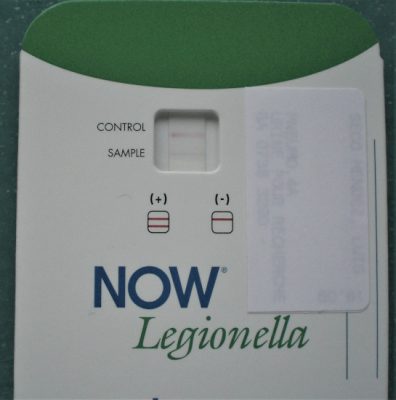
- The presence of Legionella soluble antigens in the NOW Legionella urinary test (Figure 13);
- DNA isolation using direct PCR technology on the bronchopulmonary sample;
- Isolation of bacteria cultures from pulmonary samples (sputum or bronchoalveolar lavage) then coding the isolated strain;
- Confirmation posteriori may be given if seroconversion is proven.
3. Today what is known about the epidemiology of Legionellosis in France and Europe?
Epidemiology is a branch of medical science which deals with the incidence and prevalence of a disease in large populations and the detection of the source and causative agent of such disease.
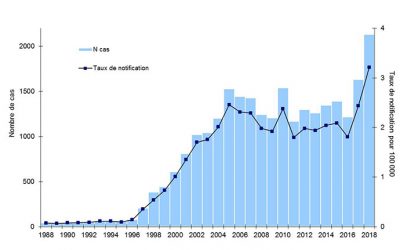
Today, different methods for data analysis are carried out, which have led to a reliable descriptive epidemiology:
- Guidelines have been implemented by the regulatory authorities which require the documentation and classification of all cases of legionellosis as “confirmed” or “probable,” according to biological test results;
- Classification of “nosocomial [10] cases” or “community cases” according to where the disease was acquired;
- Recording the spread of the disease based on the number of people affected by the same strain: isolated cases or grouped cases or epidemic cases (see Table 1); and
- Finally, cases related to travel to another country other than France are reported to the European Legionella Surveillance Network EldsNet [21].
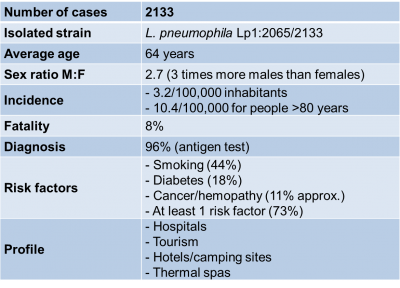
Table 1. Epidemiology data on Legionellosis cases in 2018 extracted from “Données de Santé Publique France 2019” (see Ref. [19]).
Data collected from the 2018 legionellosis epidemic in France (figures published in August 2019) highlights the different characteristics of the disease as summarized in Table 1; in its commentary Santé Publique France [22], emphasizes this clear increase: 2,133 cases and a national incidence rate of 3.2%, the highest figure recorded since surveillance began in 1988.
No particular reason was found to explain this except for a net increase in cases in June (favorable meteorological factors), numerous sporadic cases and no new risk factors.
In Europe, case notification is the responsibility of the ECDC (European Centers for Disease Prevention and Control). This monitoring activity is effective in thirty European countries. The number of reported cases has increased from 5,835 in 2013 to 9,238 in 2017 [23]. The incidence is 1.8/100,000 inhabitants.
The four countries that reported the most cases of legionellosis were: in first place Italy: 2013 cases; France in second place: 1,630 cases; Spain in third place: 1,363 cases; and Germany in fourth place: 1,280 cases. But these figures should be interpreted with caution as not all of the countries use the most recent diagnostic methods. However, the present tests used and the monitoring systems in place are considered very important for the accuracy of the data.
Note that for the USA, the annual incidence rate in 2014 was 1.62 cases per 100,000 inhabitants, an increase from 0,42 cases reported since 2000.
4. Is it possible to prevent legionellosis?
4.1. How can we monitor water quality systems?
There are no vaccines that can prevent legionellosis! Therefore, the entire prevention strategy is based on individual or collective measures.
Prevention measures targeting domestic hot water and cooling tower systems are based on several fundamental regulatory texts [24] : Guide for investigation and management assistance of the High Council for Public Health, July 11, 2013 – Decree of February 1st, 2010 – Circular of December 23, 2010 – Decree of December 14, 2013.
The general guidelines regulating the use of domestic hot water (see the monitoring sheet published by the Ile-de-France Regional Health Agency) are:
Whether it be for individual or collective use, it is necessary to continually control the temperature of hot water storage tanks, which must always be:
- above 55°C at the end of production;
- above 50°C at any distribution point;
- The temperature of cold water must be below 20°C.
Regular maintenance and monitoring of hot water installations must be ensured by removing stagnant water in the piping system, checking for the presence of corrosion in the pipes and by descaling taps and shower heads, as well as rectifying poor maintenance practices of the water supply network.
Individual prevention “at home.” In addition to the above rules, there are two further important precautions to take:
- After a prolonged absence from a property (holiday vacation, for example), it is essential to let run all hot water taps and shower heads for several minutes.
- Be cautious with rarely used water points, as they are potential reservoirs of Legionella.
Risky installations are classified into two groups according to their anthropogenic activity:
Group A: installations at high risk of Legionella dispersal:
- A1: over an area of one or more municipalities (measuring several hundred square meters or several square kilometers).This is the case for roof-mounted cooling towers installed in buildings or industrial sites, for air conditioning requirements. Contamination is caused by the release of hot water vapor into the atmosphere. To prevent such contamination, the cooling towers must be regularly maintained, cleaned and disinfected using biocides.
- A2: several square meters around communal water sources: collective domestic hot water systems.
Group B:
- B1: collective water-misting equipment, spas, vehicle washers, lagoon systems for wastewater treatment plants, professional high-pressure cleaners.
- B2: equipment used in oxygen therapy, sleep apnea, nebulization, dental care.
4.2. How do we know that the water network is contaminated?
Research into water network systems is well codified by the AFNOR Standard NF T 90-431: “Water Quality. Search for and enumeration of Legionella spp. and Legionella pneumophila.” This is a method by direct inoculation and after concentration. It applies to all types of water: both clean and dirty. Since 2010, the NF T90-471 standard using detection by molecular biology type PCR has existed: this technique is carried out in the case of a specific request by the health authorities.
4.3. What are the obligations of public institutions?
For these institutions, the decree of February 1st, 2010, and the circular of December 21, 2010 (see ref. [19]) must be applied in order to prevent contamination of the domestic hot water systems. They relate to the monitoring of Legionella in facilities dealing with the supply, storage and distribution of domestic hot water in healthcare institutions, medical social facilities and prisons, hotels and tourist residences, campsites and other establishments which are open to the public.
Other guidelines, which can be consulted on the Internet, apply specifically to all types of establishments open to the public, from campsites to hotels:
- Legionella monitoring of spas and pools,
- Legionella monitoring of thermal springs,
- Legionella monitoring of car washers,
- Legionella monitoring of tourist establishments,
- Legionella monitoring of cooling towers (January 20, 2014),
- Recommendations for residential buildings.
Regarding hospitals, the distribution of circulars from the Direction Générale des Soins and the Conseil Supérieur d’Hygiène de France have helped healthcare professionals to understand the risks for hospitalized patients and have led to the development of a response guide. In the field, the Committees for the Control of Nosocomial Infections (CLIN) and the Hygiene Units have developed procedures, including the establishment of a “water quality monitoring logbook” [25].
4.4. What to do in case of contamination?

If the water system is contaminated with Legionella, the system operator must immediately take the appropriate measures according to risk levels [26].
For a simple disinfection intervention, various processes are applied, used alone or in combination. They can be:
- Mechanical: pipe descaling or repair;
- Physical: in particular thermal shock. Used only in hot water systems, it consists in maintaining the water temperature at 70°C for at least 30 minutes. This procedure has been used in medium-sized hospitals, but during the heating process, no one can use the water and it requires the presence of personnel at each point-of-use to avoid burns; or
- Chemical: these are highly controlled chemical agents, the main ones of which are chlorinated compounds that generate hypochlorites.
5. Messages to remember
- During the 1970s and 1980s, three major infectious diseases of bacterial origin emerged: (1) Borrelia burgdorferi agent of Lyme disease, (2) Helicobacter pylori responsible for gastric ulcers, and (3) Legionella pneumophila and Legionellosis.
- Each time, it was chance circumstances that led to the discovery of the causative bacteria. In fact, they had existed for a long time, but had simply been ignored!
- Legionella most certainly existed in environmental waters long before the Philadelphia outbreak, but they were not well known because they had not yet had an opportunity to infect humans.
- It is we humans with our hot water demands and its multiple uses for comfort, pleasure or industry requirements, that has given rise to a new and totally unpredictable disease.
- As Legionella is ubiquitous in environmental freshwater, its eradication in the environment is impossible.
- It is the responsibility of all of us to promote and enforce strict rules for the proper use of hot water and the prevention of its contamination; rules that must, above all, be put into practice in order to protect the people most “at risk”.
The author would like to thank Mrs. Krysha Marca for her invaluable help in the translation in english of the original article in french.
Notes and references
Cover image. Some press coverage.
[1] Fraser DW, Tsai TR, Orenstein W. et al Legionnaires’ disease: description of an epidemic of pneumonia. N. Engl. J. Med. 1977, 297, 1189-1197
[2] McDade JE, Shepard CC, Fraser DW et al. Legionnaires’ disease. Isolation of a bacterium and demonstration of its role in other respiratory disease. N. Engl. J. Med. 1977, 297, 1197-1203
[4] Thomas G. & Morgan-Witts M., Trauma – The search for the cause of Legionnaires’ disease. Arrow Books, 1981; Thomas G. & Morgan-Witts M., Trauma – In search of a murderous virus, French translation by Destanque P., Ed. Encre 1982
[5] Brenner DJ, Steigerwalt A.G. McDade J.E. Classification of the Legionnaires’ Disease bacterium: Legionella pneumophila, genus novum, species nova of the family Legionellaceae, familia nova. Ann. Intern. Med. 1979, 90, 656-658.
[6] Moliner C., Ginevra C., Jarraud S. et al. Rapid identification of Legionella species by mass spectrometry. J. Med. Microbiol. 2010, 59,273-284;
[7] Mish EA. Legionella: virulence factors and host response. Curr. Opinions. Infect. Dis. 2016, 29, 280-286
[8] Gomez-Valero L., Rusniok C., Carson D. et al. More than 18,000 effectors in the Legionella genus genus provide multiple, independent combinations for replication in human cells. Process Nat. Acad. Sci. USA 116 (6) 2265-2273; first published January 18, 2019 https://doi.org/10.1073/pnas.1808016116
[9] Fliermans C.B., Cherry W.B., Orisson L.H., Smith S.J., et al. Ecological distribution of Legionella pneumophila. Appl. Environm. Microbiol. 1981, 41, 9-16
[10] Orisson L.H., Cherry W.B., Fliermans C.B. et al. Characteristics of environmental isolates of Legionella pneumophila. Appl. Environm. Microbiol. 1981, 42, 109-115
[11] Molmeret M. et al. Amaoebae as training grounds for intracellular bacterial pathogens. Appl. About. Microbiol. 2005; doi:10.1128/AEM.71.1.1.20-28.2005
[12] Lau H.Y. &Ashbolt N.J. The role of biofilm and protozoa in Legionella pathogenesis: implications for drinking water. J. Appl. Microbiol. 2009,107,368-378; Steinert M., Hentschel U.& Hacker J. Legionella pneumophila: an aquatic microbe goes astray. FEMS Microbiol. Rev. 2002, 26, 149-162; Declerck P. Biofilms: the environmental playground of Legionella pneumophila. Env.Microbiol.2010, 12, 557-566; Abdl-Nour, Duncan C, Low D.E et al . Biofilms: the stronghold of Legionella pneumophila. Int J. Mol. Sci. 2013, 14, 21660-21675
[13] Dietersdorfer E., Kirschner A., Schrammel B. et al Starved viable but non-culturable (VBNC) Legionella can infect and replicate in amoebae and human macrophages. Water Res. 2018
[14] Fields B.S., Benson R.F. & Besser R.E. Legionella and Legionnaires’ Disease: 25 years of investigation. Clin. Microbiol. Rev. 2002, 13, 506-526
[15] Cunha B.A., Burillo A.&Bouza E. Legionnaires’ disease. Lancet 2016, 387, 376-385
[16] McCormick J.B. & Fisher-Hoch S. 1997. Chasseurs de virus. Presses de la Cité, 498 pages
[17] Jarraud S., Reyrole M., Meugnier H. et al Légionellose. Presse Med 2007, 36, 279-87; Jamilloud Y., Jarraud S., Lina G., et al. Legionella, legionellosis, 2012, Med Sci (Paris) 28, 639-45
[18] ANSM : Agence Nationale de Sécurité des Médicaments et des produits de Santé (replaces AFSSAPS), a french agency
[19] Assessment of legionellosis cases that occurred in France in 2018 (August 2019) Santé Publique France. https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-et-infections-respiratoires/legionellose/articles/bilan-des-cas-de-legionellose-survenus-en-france-en-2018 (in french)
[20] Centre National de Référence des Légionelles (french National Reference Centre for Legionella). http://cnr-legionelles.univ-lyon1.fr/
[21] ELDSNet : European Legionnaires’ Disease Surveillance Network, coordinated by ECDC (European Center for Disease Prevention and Control), created in 2005. EWGLI (European Working Group for Legionella Infections), created in 1986, will become EWGLINET and then in 2010 becomes ELDSNet.
[22] Santé Publique France. Since 1 May 2016, it has included: INVS (Institut National Veille Sanitaire), Inpes (Institut national de prévention et d’éducation pour la santé), Eprus (Etablissement de préparation et de réponse aux urgences sanitaires).
[23] European Centre for Disease Prevention and Control, Legionnaires’ disease. In: ECDC. Annual epidemiological report for 2017 (2019), Stockholm.
[24] https://www.hcsp.fr notices and reports/guide for investigation and case management assistance in legionellosis, https://www.iledefrance.ars.sante.fr/la-legionellose (in french)
[25] Cheron J. (2006) Maîtriser le risque légionelles. Ed. Johanet, 325 p. (in french)
[26] Prevention of legionellosis: obligations by type of installation and establishment (in french)
The Encyclopedia of the Environment by the Association des Encyclopédies de l'Environnement et de l'Énergie (www.a3e.fr), contractually linked to the University of Grenoble Alpes and Grenoble INP, and sponsored by the French Academy of Sciences.
To cite this article: CROIZE Jacques (January 5, 2025), “The Philly Killer”: Emergence of Legionella pneumophila, a ubiquitous environmental bacterium, Encyclopedia of the Environment, Accessed January 30, 2025 [online ISSN 2555-0950] url : https://www.encyclopedie-environnement.org/en/health/philly-killer-legionellosis/.
The articles in the Encyclopedia of the Environment are made available under the terms of the Creative Commons BY-NC-SA license, which authorizes reproduction subject to: citing the source, not making commercial use of them, sharing identical initial conditions, reproducing at each reuse or distribution the mention of this Creative Commons BY-NC-SA license.