Collembola: actors of soil life
PDF
An unsuspected diversity of invertebrates swarms under our feet when we walk on the ground in a forest, meadow or garden. Invisible communities are active in the soil as in a parallel world, with the difference that this world is very real and well connected to the above ground level. It is indeed essential to plants that grow above it. Among the invertebrates living in the soil, Collembola (or springtails) are important because of their abundance and therefore their ability to impact the functioning of an entire ecosystem. They have a wide variety of forms and live in a wide variety of habitats. Their main role is to regulate the microorganisms responsible for the decomposition of organic matter and the recycling of nutrients that will be used by plants for their development. Unfortunately, many human activities can affect the communities of Collembola. These include, for example, soil pollution by metals, pesticides, etc., but also human practices such as the introduction of exotic plants or the use of waste to fertilize the soil.
1. Soil invertebrates, shadow workers
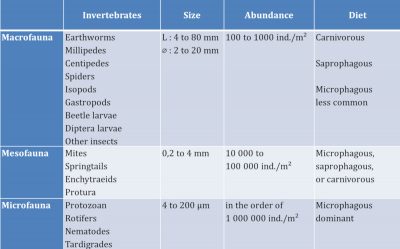
From the point of view of general ecological characteristics, macrofauna and mesofauna invertebrates have terrestrial life. Small white grubs, cousins of earthworms, potworms, have an aquatic life – like microfauna invertebrates – because they live in the water retained in the pores of the soil. These invertebrates have developed strategies to resist dryness in dry periods such as slowed life or encysting.
The major role of soil fauna is to contribute to the decomposition and mineralization of organic matter, thus ensuring the circulation of nutrients (nitrogen, phosphorus, potassium, etc…) and their availability for plant development at the surface. Soil structuring is another fundamental action of soil invertebrates.

All these activities facilitate the circulation of water and air in the soil. They thus improve the water regime and soil aeration. They also stabilize its structure, which is generally beneficial to plants.

In the following sections we will get to know one of these wildlife groups, the Collembola. Like mites, Collembola (or springtails) are small arthropods that are very abundant in the soil. They contribute to the functioning of terrestrial ecosystems. However, unlike mites or earthworms, their names are generally unknown to the general public.
2. Collembola, a diversity of forms and habitats

- the furca, which is a jumping element operating as a spring lever;
- the ventral tube that allows them to regulate their ionic and hydric balance by absorbing water from the soil with the ions it contains.
There are four orders of Collembola that differ in morphology:
- Entomobryomorpha (cylindrical body, segmented with long appendages);
- Poduromorpha (cylindrical body, segmented, with short appendages);
- Symphypleona (spherical bodies with long appendages);
- Neelipleona (spherical bodies with short antennas);
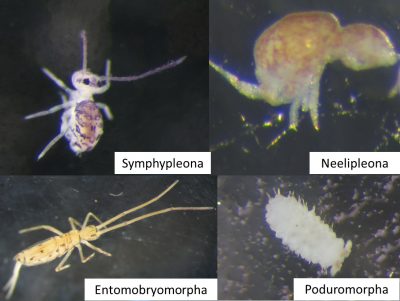
Collembola species have morphological and physiological adaptations to habitat depth and closure:
- Large, pigmented species with highly developed locomotor organs (legs, furca; e.g. Entomobryomorpha, Figure 4) and sensory organs sensitive to air (sensory silk) and light (eyes) are selected in open environments (e.g. meadow) and on the soil surface [4].
- In the forest, and at depth in the soil, small, blind, unpigmented or poorly pigmented species with small locomotor appendages dominate (e. g. Poduromorph, Figure 4). The latter often also have a particular sensory organ, sensitive to chemical molecules, the post-antennal organ that compensates for the absence of other sensory organs.
Most species feed on microorganisms (fungi, terrestrial microalgae, bacteria), most often fungal filaments. Others consume dead plant organs, or excreta from other invertebrates. Others pierce the walls of plants and fungi and suck up the liquids they contain. Finally, a very small proportion is predatory of Nematodes, Rotifers, Tardigrades or other Collembola. It is essentially through their trophic activity, i.e. their search for and consumption of food, that Collembola will perform different functions in the soil and this is what we will discover in the following section.
3. Collembola, small but active
Like most soil wildlife stakeholders, Collembola have a direct and indirect effect on the decomposition of organic matter and the recycling of nutrients.
Some species, by consuming dead plant organs (leaves, root needles, etc.), or excreta from other invertebrates, contribute to the fragmentation of dead plant matter and the mineralization of organic matter, as well as to the surface structure of the soil (OF and OH layers of humus, see Figure 2).
However, a larger part of the fragmentation of dead plant organs is exerted by macrofauna, and mineralization is largely (70-80%) provided by microorganisms. It is therefore mainly indirectly that the activity of collembola on the mineralization of organic matter and the recycling of nutrients will be carried out, by regulating soil microorganisms (bacteria and fungi):
- By consuming microorganisms in a moderate way, Collembola stimulate the growth of their populations and consequently the mineralization of organic matter. But depending on the species present, the filaments of the fungi can be consumed in excess by Collembola, thus preventing the excessive development of certain species, in particular pathogenic fungi.

Figure 5. Heteromurus nitidus (Entomobryomorpha, Entomobryidae) with bristles on the body capable of fixing and transporting fungal spores. [Source: Photo © Sandrine Salmon] - Collembola also spread fungal spores and bacteria, either through their intestinal transit or by attaching them to the body’s bristles (Figure 5).
- Finally, their faecal pellets provide a favourable habitat for the development of microorganisms.
By consuming phytopathogenic fungi, Collembola can limit fungal diseases in plants [5]. By stimulating the development and activity of mycorrhizal fungi, they can also promote the absorption of phosphorus by cultivated plants or regulate the root architecture of certain plants.
Finally, although the activity of springtails is generally beneficial to humans and crops, it is important to note the existence of two species considered harmful because they consume cultivated plants (usually alfalfa and clover seedlings). These are Sminthurus viridis and Bourletiella hortensis. But Sminthurus viridis, originated from Europe, is mainly a pest in alfalfa crops in Australia, where it has been accidentally introduced by humans and where it probably does not have a predator [3].
4. How do human activities disrupt Collembola?
The anthropogenic disturbances that can affect springtail communities are so diverse and varied that not all of them can be addressed in this article. Most often, it concerns soil contamination by pollutants such as metals in high concentrations, pesticides, polycyclic aromatic hydrocarbons (PAHs)…. Human practices such as the introduction of exotic plants into a country, the use of waste to fertilize the soil, soil compaction or forest fragmentation can also be harmful, not to mention of course the destruction of soil caused by urbanization. And no! Collembola don’t live in concrete!
4.1. Effect of metals
Soils polluted by metals are very diverse. Examples include former marshalling yards (cadmium, copper, nickel, lead, zinc), former foundries (iron, zinc, cadmium, copper) or former mines (silver, lead, zinc…) [6], but also agricultural soils (copper) [7] or urban parks (cadmium from road traffic). Soil pollution by high concentrations of metals can reduce the abundance and diversity of springtails. In all cases, it modifies communities, i.e. the species usually present are replaced by others. This generally results, at the regional scale, in a high degree of homogenization of communities, which are then dominated by a small number of metal-tolerant species. The risk of this homogenization of communities is the loss of biodiversity at the regional scale.
4.2. Effects of waste
The use of organic waste can be very beneficial for fertilizing agricultural soils, forest plantations or for the remediation of degraded soils. This type of amendment improves soil structure and water retention, and increases the concentration of nutrients and organic matter. This waste can be either agricultural waste of plant (compost) or animal (manure) origin, sludge from sewage treatment plants or waste of industrial origin, or a mixture of these various wastes. The problem is that these wastes can ultimately prove to be unfavourable to communities of springtails, or even very harmful since they can greatly reduce the abundance of certain species and their reproduction rate. They often have metal or ammonium concentrations high enough to become toxic, even when metal levels are below legal standards [8].
4.3. Effect of pesticides
Collembola are often the object of collateral damage resulting from the treatment of crops with insecticides to eradicate insect pests. Thus, it has already been shown that the application of insecticides to fields in conventional agriculture causes a decrease in the abundance and diversity of Collembola. There is also a change in community structure due to the fact that some species are more sensitive than others [9]. A study of the features of collembola (eye development, furca, antennae, pigmentation and silks) showed that species adapted to depth are the most vulnerable. This is probably because these species cannot escape their environment when treatments are applied, unlike surface species that can gain refuge and then return. It should also be noted that compaction of agricultural soils can be as harmful to soil invertebrates as insecticide pollution.
4.4. Effects of “alien” plant species
A species is said to be exotic when it is observed in a geographical area from which it does not originate, unlike native plants (see When invasive plants also wander in the fields). Alien species are generally introduced by humans into a geographical area that is not part of their natural range. These species have therefore not co-evolved with other species that inhabit the same environment, which can cause a disruption of the ecosystem where they are introduced. For example, they can cause local extinction of native species. The invasion of a habitat by exotic plant species has already shown significant effects on soil characteristics such as a change in the quantity and quality of organic matter [10].
They can also directly impact animal communities in the soil. Thus, the planting of Eucalyptus to replace Oaks in Portugal has led to a decrease in the species richness of Collembola and the replacement of specialist species by generalist species capable of colonising a large number of environments. The planting of Pinus radiata in Australia [11] has also resulted in the replacement of several native springtail species with exotic springtails.
4.5. Consequences of the homogenization of communities and the decrease in specific wealth
All these factors that alter springtails communities are also harmful to other soil invertebrate groups.
When the abundance and/or diversity of fauna decreases, it can no longer properly perform its functions such as nutrient recycling or pathogen regulation (see section 3). Indeed, the functions performed by soil organisms are spread over a large number of species. When several species disappear, the functions that these species used to perform are no longer performed. This can affect the functioning of the ecosystem. For example, if nutrient recycling is not done properly, it becomes difficult for plants to grow on the surface, which ultimately leads to soil erosion.
Also, the replacement of local species by generalist species and the resulting large-scale homogenization of communities alter the resistance and resilience of ecosystems (see What is Biodiversity?). In other words, in the event of a disturbance of the ecosystem, it will not be able to resist or return to its original state. Let us take as an example of disruption the arrival of a phytopathogenic fungus. If the fungivorous species that feed on this fungus have disappeared from the ecosystem, its development is no longer regulated, and the plant species attacked can be completely decimated.
Consequently, we must protect our soils by limiting urbanization, pollution (by pesticides, excess fertilizers, waste, metals), and soil compaction (by deep and intense ploughing or skidding equipment).
Acknowledgements to Jean-François Ponge, Professor Emeritus at the Muséum National d’Histoire Naturelle, and to Charlotte Fromont, my daughter, for proofreading this text.
References and notes
Cover image. Two specimens of Caledonimeria mirabilis, a species of collembola endemic to New Caledonia. [Source: Photo © Cyrille d’Haese]
[1] Gobat J.-M., Aragno M. & Matthey W., 2003. Le sol vivant. Bases de pédologie – Biologie des sols, 2nd ed. Presses Polytechniques et Universitaires Romandes, Lausanne. (in french)
[2] Frans Janssens, http://www.collembola.org/
[3] Hopkin S.P., 1997. Biology of the springtails (Insecta: Collembola). Oxford University Press, Oxford.
[4] Salmon S., Ponge J.F., Gachet S., Deharveng L., Lefebvre N. & Delabrosse F., 2014. Linking species, traits and habitat characteristics of Collembola at European scale. Soil Biology & Biochemistry 75, 73-85.
[5] Schrader S., Wolfarth F. & Oldenburg E., 2013. Biological control of soil-borne phytopathogenic fungi and their mycotoxins by soil fauna: a review. Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca Agriculture 70, 291-298.
[6] Russell D.J. & Alberti G., 1998. Effects of long-term, geogenic heavy metal contamination on soil organic matter and microarthropod communities, in particular Collembola. Applied Soil Ecology 9, 483-488.
[7] Santorufo L., Cortet J., Nahmani J., Pernin C., Salmon S., Pernot A., Morel J.L. & Maisto G., 2015. Responses of functional and taxonomic collembolan community structure to site management in Mediterranean urban and surrounding areas. European Journal of Soil Biology 70, 46-57.
[8] Renaud M., Chelinho S., Alvarenga P., Mourinha C., Palma P., Sousa J.P. & Natal-da-Luz T., 2017. Organic wastes as soil amendments: effects assessment towards soil invertebrates. Journal of Hazardous Materials 330, 149-156.
[9] Chelinho S., Domene X., Andres P., Natal-da-Luz T., Norte C., Rufino C., Lopes I., Cachada A., Espindola E., Ribeiro R., Duarte A.C. & Sousa J.P., 2014. Soil microarthropod community testing: a new approach to increase the ecological relevance of effect data for pesticide risk assessment. Applied Soil Ecology 83, 200-209.
[10] Maurel N., Salmon S., Ponge J.F., Machon N., Moret J. & Muratet A., 2010. Does the invasive species Reynoutria japonica have an impact on soil and flora in urban wastelands? Biological Invasions 12, 1709-1719.
[11] Greenslade P., 2007. The potential of Collembola to act as indicators of landscape stress in Australia. Australian Journal of Experimental Agriculture 47, 424-434.
The Encyclopedia of the Environment by the Association des Encyclopédies de l'Environnement et de l'Énergie (www.a3e.fr), contractually linked to the University of Grenoble Alpes and Grenoble INP, and sponsored by the French Academy of Sciences.
To cite this article: SALMON Sandrine (May 2, 2019), Collembola: actors of soil life, Encyclopedia of the Environment, Accessed April 19, 2025 [online ISSN 2555-0950] url : https://www.encyclopedie-environnement.org/en/life/collembola-actors-of-soil-life/.
The articles in the Encyclopedia of the Environment are made available under the terms of the Creative Commons BY-NC-SA license, which authorizes reproduction subject to: citing the source, not making commercial use of them, sharing identical initial conditions, reproducing at each reuse or distribution the mention of this Creative Commons BY-NC-SA license.




