原油:其生物起源的证据
“被告请起立!说出你的姓名、年龄和出身。”
如果必须在这样一个假想的法庭上出庭的话,“岩石油脂”、“古老”、“生物的”这些词汇将回答世界上所有关于原油的问题。对于石油的起源,科学家持不同的观点。一些科学家认为石油起源于矿物,因此具有非生物来源。然而,众多迹象表明,石油起源于生物,也就是说,它与几百万年前的生物有关。石油在世界各地留下了与生命相关的痕迹。本文从历史的角度回顾了石油起源于生物的各种证据。
1. 争论根源的观点和事实
1.1. 从古代哲学家到文艺复兴时期的学者

[来源:公共领域,通过维基共享](Georgius Aagricola:乔治·阿格里科拉;De ortu & caufis fubterraneorum:从地底升起;De natura eorum qux effluent ex terra:关于流出地球的事物的性质;De natura fofsilium:关于矿物的性质;De ueteribus & nouis metallis:新旧金属;Bermannus,fiue Dere metallica Dialogus:伯曼努斯,金属对话;Interpretatio Germanica uocum rei metallicae,addito Indice foeeundifsimo:金属的德语翻译,附加索引)
尽管原油或其衍生物自新石器时代以来就已被人类使用[1],但其起源长期以来却一直是个谜。罗杰·培根[2]在1268年出版的专著《第三著作》(Opus Tertium)中写道,亚里士多德[3]和其他古代历史哲学家均未讨论过石油和沥青的起源。在文艺复兴时期,出现了关于石油起源的两种相互矛盾的假设。1546年,乔治·阿格里科拉[4]出版了《在地球的自然环境中》(De Natura eorum quae Efflunt ex Terra)(图1),在这部作品中首次提到了石油这个词,它来自拉丁语petra oleum (石油)。阿格里科拉拓展了亚里士多德关于石油从地下深处喷出的概念,提出沥青是由含硫蒸汽冷凝而来。安德烈亚斯·利巴维乌斯[5]在1597年出版的《炼金术》一书中提出,沥青是由古树的树脂转化形成。此后,一场漫长的科学辩论延续开来,一方观点支持石油的非生物起源,另一方认为石油是由石化有机物转化而来的。
1.2. 首批科学家相反的观点

有趣的是,煤的生物起源从未引起过争议。煤中含有的植物化石为其生物起源提供了不可否认的证据(图2)[6]。俄罗斯科学家米哈伊尔·罗蒙诺索夫[7]将液态石油和固体沥青联系起来,并承袭了利巴维乌斯的想法,在1757年提出了一个假说,即这两种碳质化石燃料源于植物废料在温度和压力的作用下,在底层土壤中转化而来。19世纪初德国地质学家和化学家亚历山大·冯·洪堡[8]和法国热力学家路易斯·约瑟夫·盖–吕萨克[9]否定了该假说,认为石油是地球的原始化合物,通过低温喷发从深处升起。这种纯粹的化学假说并非没有论据,自20世纪中叶以来,俄罗斯学派广泛传播了石油的非生物起源理论,这种观点频繁出现,表明地球深处的石油比我们想象的要多得多。
1.3. 石油非生物起源支持者的论点

气态行星上应该不会出现生命。烃的存在表明非生物过程导致了简单烃分子的形成。土星卫星土卫六(图3)就是一个例子。土卫六的大气和表面都富含甲烷和乙烷。在坠落地球的陨石中发现了烃和相当复杂和高分子量的有机大分子。
甲烷是在地幔岩浆岩的蚀变过程中形成的。在此过程中,一些富铁矿物风化过程中产生的氢气(H2)与二氧化碳(CO2)发生所谓的萨巴捷反应。该反应在高温高压下,在镍催化剂的作用下进行,产生甲烷和水。另外,将一氧化碳(CO)和二氢合成烃的费托反应可能在岩浆冷却时发生,并通过和CH2反应产生烃。最后,碳酸铁(FeCO3)在有水的情况下发生热分解,也可以生成简单烃。
虽然不能否认非生物过程导致了烃的形成,但这些机制很难解释地球上石油的数量、多样性及位置。石油来源于沉积有机质,即生物体,这一观点已被自然观察、实验室分析和实验广泛证实。
2. 通过自然观测获得证据
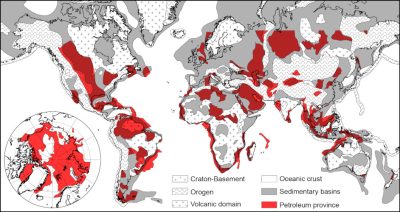
2.1. 沉积盆地中心积油
2.2. 旋光性的证据
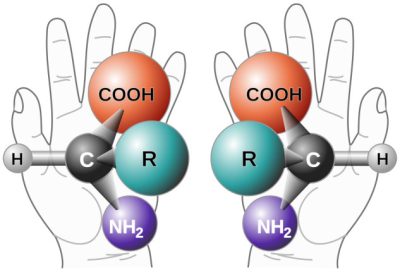
[来源:文章(πϵρήλιο)℗(手征性(Chirality with hands).jpg)[公共领域],通过维基共享。]
3. 地球化学证据
3.1. 碳同位素
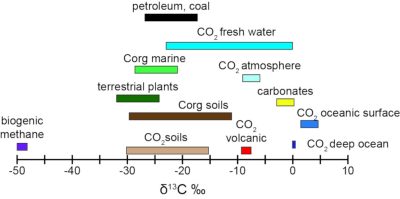
[来源:改编自特朗伯和德鲁菲尔(Trumbore & Druffel);参考文献[10]](Petroleum:石油;Coal:煤;Fresh water:淡水;Corg marine:海洋有机碳;Atmosphere:大气;Terrestrial plants:陆生植物;Carbonates:碳酸盐;Corg soils:土壤有机碳;Oceanic surface:海洋表面;Biogenic methane:生物甲烷;Volcanic:火山岩;Deep ocean:深海)
3.2. 在石油中发现卟啉
德国化学家汉斯·菲舍尔[12]因其对有机色素,特别是血液(血红素)和植物(叶绿素)中的有机色素的研究而获得诺贝尔化学奖(1930年)。
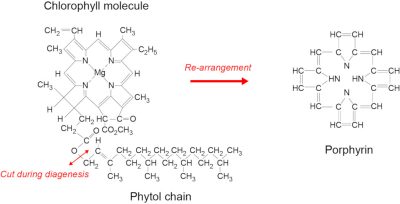
由四吡咯核和叶绿醇侧链组成。植物死亡后,叶绿素分子分裂为两部分,并根据沉降介质的条件不同而发生不同的演变。四吡咯核部重新组织,形成一种叫做卟啉的分子。卟啉有几十种类型,所有的石油都含有这些分子,这无疑证明了卟啉的生物起源。[来源:©F. 鲍丁(Baudin)](Chlorophyll molecule:叶绿素分子;Re-arrangement:重新排列;Cut during diagenesis:成岩作用中断裂;Phytol chain:植醇链;Porphyrin:卟啉)
汉斯·菲舍尔的学生阿尔弗雷德·特雷布斯[13]在1936年强调,卟啉普遍存在于原油和富含干酪根的粘土中。他指出卟啉从叶绿素转化而来的方式,从而提供了卟啉来源于植物的明确证据。阿尔弗雷德·特雷布斯因此被认为是有机地球化学之父。
3.3. 越来越多的生物标志物
自这一发现以来,我们不再计算油中检测到的分子的数量了。这些分子源自生物体中已知的某种分子,甚至与之完全相同(图8)。这些分子被称为生物标志物,是真正的地球化学化石,因为它们的结构非常接近生物体的生物分子。
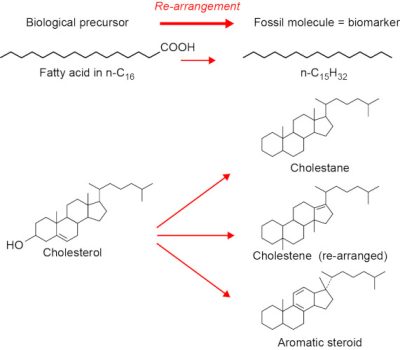
[来源:©F. 鲍丁(Baudin)](Biological precursor:母质来源;Re-arrangement:重新排列;Fossil molecule:化石分子;Biomarker:生物标志物;Fatty acid in n-C16:n-C16脂肪酸;Cholesterol:胆固醇;Cholestane:胆甾烷;Cholestene:胆甾烯;Aromatic steroid:芳香甾类)
随着时间的推移和深度温度的升高,干酪根经过天然热裂解形成石油时,其中一些生物标志物保持不变,甚至成为石油的一部分。因此,很难想象所有的石油中都存在如此多的复杂分子,而这些分子只能通过非生物过程产生。
3.4. 与浮游植物多样化共同进化的海洋石油的化学特征
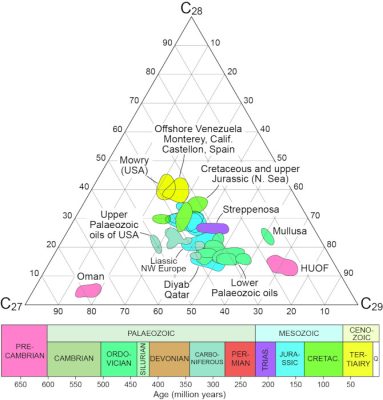
[来源:改编自格兰瑟姆和韦克菲尔德(Grantham & Wakefield);参考文献[13]](Offshore Venezuela:内瑞拉近海:Monterey,Calif 蒙特利,加利福尼亚州;Castellon,Spain:卡斯特利翁,西班牙;Mowry(USA):莫里(美国);Cretaceous and upper Jurassic (N.Sea): 白垩纪和晚侏罗系(北海);Upper Palaeozoic oils of USA:美国晚古生代石油;Streppenosa:斯特雷佩诺萨(上三叠统地层);Mullusa:穆卢萨;Liassic NW Europe:西北欧(早株罗系)里阿斯统;Oman:阿曼;Diyab Qatar:迪亚布 卡塔尔;Lower Palaeozoic oils:晚古生代油;HUOF:胡夫:Precambrian:前寒武纪;Palaeozoic:古生代;Cambrian:寒武纪;Ordovician:奥陶纪;Silurian:志留纪;Devonian:泥盆纪;Carboniferous:石炭纪;Permian:二叠纪;Mesozoic:中生代;Trias:三叠纪;Jurassic:侏罗纪;Cretac:白垩纪;Cenozoic:新生代;Tertiairy:第三纪)
20世纪80年代末,壳牌石油化工公司的地球化学家分析了6.5亿至4500万年前的海洋岩石中的400多种原油,提取并鉴定了不同种类的甾烷,并通过碳原子数将其分组。结果表明,随着地质年代的推移,拥有28个碳原子的甾烷比例在增加,而拥有29个碳原子的甾烷比例在下降,拥有27个碳原子的甾烷几乎保持稳定(图9)[14]。
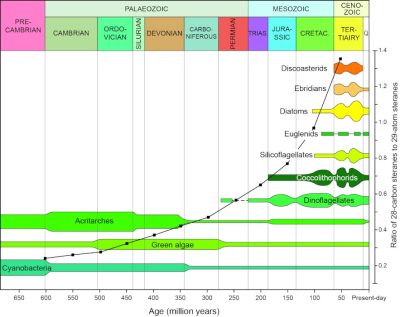
[来源:改编自格兰瑟姆和韦克菲尔德;(Grantham & Wakefield)参考文献[13]。] (Precambrian:前寒武纪;Palaeozoic:古生代;Cambrian:寒武纪;Ordovician:奥陶纪;Silurian:志留纪;Devonian:泥盆纪;Carboniferous:石炭纪;Permian:二叠纪;Mesozoic:中生代;Trias:三叠纪;Jurassic:侏罗纪;Cretac:白垩纪;Cenozoic:新生代;Tertiairy:第三纪Cyanobacteria:蓝藻细菌;Acritarches:疑源类;Green algae:绿藻类;Discoasterids:盘星藻类;Ebridians:硅质鞭毛类;Diatoms:硅藻类;Euglenids:眼虫类;Silicoflagellates:硅鞭藻类;Coccolithophorids:颗石藻类;Dinoflagellates:钩鞭藻;Ratio of 28-carbon steranes to 29-atom steranes:28-碳的甾烷和29个碳原子甾烷的比例;Age(million years):年代(百万年);Present-day:至今)
海洋石油化学特征的演变与浮游植物化学特征的进化同步进行,这显然表明了一种因果关系,并为石油的生物起源提供了证据。
4. 通过实验证明
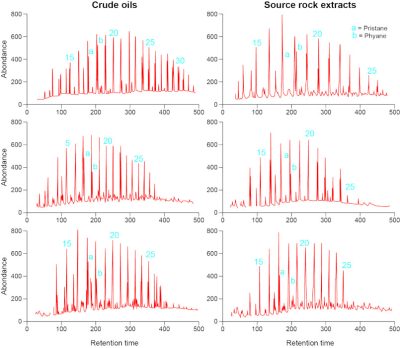
在20世纪下半叶,随着分析技术的发展,在实验室中可以重建沉积盆地深部的温度和压力条件。甚至有可能在有水的情况下进行这些实验,水分子在陆地岩石中含量非常丰富。在这些实验中获得的产品在物理和化学性质上与天然石油非常相似。分析其分子组成,天然石油中的分子与加热干酪根产生的分子在性质和丰度上都非常相似(图11)。它们如此相似,地球化学家甚至认为这些分子分布有点像指纹或DNA,并以此从基因上把天然石油和它的源岩联系起来。
5. 要记住的信息
- 大量证据表明石油来源于生物,包括直接来自活生物体合成的分子,无论是原核生物还是真核生物的分子。
- 因此,卟啉这一石油中无处不在的分子来自不同类型的叶绿素。
- 同样,原油中的化石分子很容易附着在其他色素(例如类胡萝卜素)或构成原核生物或真核生物细胞壁的分子上。
- 在石油分子中,12C的比例大于13C,这一所谓的同位素特征支持了石油起源于生物的观点。因为在相同的比例下,生命选择轻同位素。
- 最后,99%以上的油田位于沉积盆地,即沉积物沉积在曾经存在生命的古代海洋或湖泊底部,如矿化的化石所示。
- 一般来说,油源岩主要含有源自海洋或湖泊浮游植物的有机物,并或多或少受到了细菌的改造。
参考资料及说明
封面图片:天然石油渗流。[来源:© F.伯杰拉特]
[1] 我们知道一种新石器时代的石斧是用沥青作为“胶水”压制而成。
https://www.franceculture.fr/emissions/lessai-et-la-revue-du-jour-14-15/le-bitume-dans-lantiquite-revue-archeopages(法语广播节目)
[2] 罗杰·培根(1214年-1294年),英国哲学家、科学家和炼金术士,科学方法之父之一。
[3] 亚里士多德(公元前384年–公元前322年),古希腊哲学家。所学之识几乎涵盖他那个时代所有的知识领域:生物学、物理学、形而上学、逻辑学、诗学、政治、修辞,偶有涉及经济学。
[4] 格奥尔格乌斯·阿格里科拉,本名格奥尔格·帕夫尔(1494年–1555年),16世纪德国科学家、矿物学和冶金学之父。
[5] 安德烈亚斯·利巴维乌斯(1555–1616),本名安德烈亚斯·利博,德国化学家和医生。1597年出版的《炼金术》是第一本系统化学著作。
[6] 煤碳沉积的形成始于石炭纪,当时大量所谓的高等植物碎屑(树木、蕨类植物……)堆积在缺氧的浅水层(泥炭型环境)中。这些条件使部分有机物逃脱分解者的作用。数百万年来,这些植物碎屑的积累和沉积导致煤层温度、压力和氧化条件逐渐发生变化,从而形成了含碳量日益丰富的化合物:泥炭(50至55%)、褐煤(55至75%)、煤炭(75至90%)和无烟煤(>90%)。大多数煤的石油潜力较低。另一方面,它们在成熟时会产生气体,特别是甲烷,这是煤矿燃烧的原因。
[7] 米哈伊尔·瓦西里耶维奇·洛莫诺索夫(1711–1765),化学家、物理学家、天文学家、历史学家、哲学家、诗人、剧作家、语言学家、斯拉夫语言文学家、教师和俄罗斯镶嵌艺术家。
[8] 亚历山大·冯·洪堡(1769年–1859年),德国博物学家、地理学家和探险家。法国科学院副院士和巴黎地理学会会长。他在探险中进行了高质量的勘测,为科学探索奠定了基础。
[9] 路易斯·约瑟夫·盖伊·卢萨克(1778年-1850年),法国化学家和物理学家,以研究气体的特性而闻名。
[10] 放射性碳14C最初存在于石油起源化合物(由光合作用形成)中,其比例与当时生活的光合作用生物中的比例相同。然而,由于该元素的地质尺度(5700年)相对较短,当前的石油不再包含14C,因此无法通过这种技术确定其年代。目前,该特性用于区分造成空气污染的颗粒物,例如来自石油产品(汽油、柴油)的颗粒物和来自木材燃烧产生的颗粒。
[11] Trumbore S.E. & Druffel E.R.M. (1995) Carbon isotopes for characterizing sources and turnover of nonliving organic matter. In R. G. Zepp & C. Sonntag (Eds.), Role of Nonliving Organic Matter in the Earth’s Carbon Cycle (pp. 7-22). Chichester: John Wiley & Sons Ltd.
[12] 汉斯·菲舍尔(1881年-1945年),德国化学家,专门从事有机化学。
[13] 阿尔弗雷德·特雷布斯(1899–1983),德国有机化学家、有机地球化学先驱。
[14] Grantham P.J. & Wakefield L.L. (1988) Variations in the sterane carbon number distributions of marine source rock derived crude oils through geological time. Organic Geochemistry,12-1,61-73.
环境百科全书由环境和能源百科全书协会出版 (www.a3e.fr),该协会与格勒诺布尔阿尔卑斯大学和格勒诺布尔INP有合同关系,并由法国科学院赞助。
引用这篇文章: BAUDIN François (2024年3月12日), 原油:其生物起源的证据, 环境百科全书,咨询于 2025年4月5日 [在线ISSN 2555-0950]网址: https://www.encyclopedie-environnement.org/zh/vivant-zh/oil-evidence-biological-origin/.
环境百科全书中的文章是根据知识共享BY-NC-SA许可条款提供的,该许可授权复制的条件是:引用来源,不作商业使用,共享相同的初始条件,并且在每次重复使用或分发时复制知识共享BY-NC-SA许可声明。








