The oyster, the sentinel of a coastline to be preserved
PDF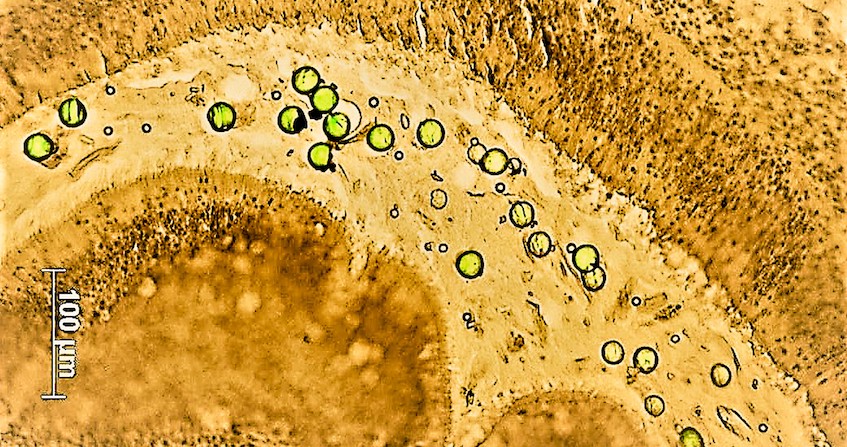
In the category of marine molluscs, the oyster is an essential species of our French coasts and has strong economic and heritage stakes. An ingenious species, it provides many services in coastal ecosystems. However, oysters undergo strong pressures, including pollution of human origin. Among these pollutions, we will detail in this article, chemical pollutions, biological pollutions such as natural toxic microalgae whose proliferation can have an anthropic origin, and new chemical and particulate contaminants, microplastics, thus completing the previous article showing the ecological importance of oysters and the threats related to global warming which weigh on them (See Oysters: these unknown architects of coastal environments). The oyster, as a sentinel species indicating the state of health (or degradation) of the coastal ecosystem, is therefore in the position of an alarm bellwether requiring strong measures to protect coastal habitats.
1. The oyster: an essential and threatened marine mollusc

2. Oysters are victims of all the evils
Overexploitation, habitat destruction, and the presence of diseases or parasites, some of them being accidentally introduced by human activity, are real scourges for oysters (See Oysters : the unknown architects of coastal environments). Recent studies show that changes linked to coastal urbanization, global warming and ocean acidification could affect oyster reproduction with consequences for the oyster industry [1]. Moreover, coastal areas, which are the closest to human activities, gather a quantity of pollutants such as pesticides, drug residues, heavy metals and hydrocarbons.
For example, in the Arcachon basin at the beginning of the 1980s, recruitment (i.e. the abundance of Crassostrea gigas oyster larvae) had become null and the renewal of natural stocks was threatened. It was only at the end of a multi-year research program that researchers demonstrated the responsibility of tributyltin [2] in antifouling paints [3] in the mortality of young larvae.
To this long list, we should add biological pollution from pathogenic bacteria and viruses, or toxic micro-algae.
2.1. Toxic micro-algae
A bloom of marine micro-algae is defined by the very rapid multiplication of these photosynthetic unicellular organisms, favoured by environmental conditions (Figure 2). It is particularly favoured and amplified by eutrophication [4] linked to discharges from urban areas and intensive agriculture, known in Brittany to stimulate green tides (see Nitrates in the environment & Phosphorus and eutrophication). However, among the thousands of species of microalgae, which are essential because they are at the base of the marine food chain and produce part of the oxygen we breathe, at least 300 are known to be toxic when they proliferate. These are known as Harmful Algal Blooms (HABs).
In recent decades, the number, intensity and geographical distribution of HAB events have been increasing, partly due to global change and eutrophication of coastal areas. Some of them are harmful because of the mass effect produced during blooms. Indeed, extreme variations (temperature, salinity) during the 2018 and 2019 heat waves in the Thau lagoon modified the planktonic communities by favouring very small species that are not
assimilated by the oyster, which generated a green water phenomenon. The origin was a bloom of Picochlorum, a phytoplankton of a few micrometres that tolerates great environmental variations but has no food interest for the oyster. These micro-algae literally suffocate the environment where they proliferate. This can lead, for example, to occlusion of the gills in filter-feeder animals or deprive surrounding species of oxygen and cause massive deaths by anoxia.
There are, moreover, a hundred of toxic micro-algae that produce phycotoxins. Some of them present a danger for human health by their accumulation in marine organisms consumed by humans. Several types of toxins are listed and classified according to the syndromes they cause in humans, including PSP (paralytic), DSP (diarrheic), ASP (amnesic), NSP (neurotoxic), and CFP (Ciguatera).

analyses the concentration of ASP, DSP and PSP toxin-producing microalgae and the level of toxins in several shellfish species along the coastline. This network provides local authorities with a decision-making tool, namely the prohibition by prefectural decree of the recreational fishing, and the fishing and sale of shellfish when the toxin content in the shellfish exceeds the health threshold established at the European level.
In France, blooms of paralytic toxin-producing micro-algae, in particular the dinoflagellates Alexandrium minutum and Alexandrium catenella, have been regularly recorded since the 1980s. In the Bay of Brest, several A. minutum blooms have occurred since the summer of 2012, whereas they had never been recorded before [3]. In July 2012, the concentration of A. minutum even reached record levels, causing the closure of the sale of shellfish exceededing the health toxin threshold by 10 times.
2.2. Chemical pollution
The oyster is used as a sentinel of environmental contamination in the framework of the chemical contamination observation network (ROCCH) operated by the French Research Institute for Exploitation of the Sea (Ifremer). Like mussels and other organisms, this mollusc has the property of concentrating some of the chemical pollutants present in its environment in proportion to its exposure. Since 1974, within the framework of the ROCCH, metals such as cadmium, copper, mercury or lead, polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDEs) and certain pesticides such as lindane, aldrin and DDT residues have been analysed once a year in the flesh of oysters in order to provide information on the quality of the marine environment.
This approach is not specific to France and in many countries of the world, oysters are used to evaluate the chemical contamination of the environment either within the framework of perennial monitoring networks or during specific scientific studies. The latter allow the search for emerging pollutants, as in a recent study carried out on the Portuguese coast in which, in addition to the pollutants described above, 29 flame retardants, 35 pesticides including pyrethroid insecticides and 13 care products such as musks and UV filters were measured [8].
Beyond its role as a sentinel species, the oyster is also a model species in environmental toxicology, a discipline that studies the effects of pollutants on organisms:
- Short-term exposures to plant protection products (at concentrations similar to those found in the marine environment) alter their DNA structure and repair processes. These effects can reach the germ cells and be transmitted to the offspring [9].
- Pesticides also affect the oyster’s immune defences and metabolism, especially energy metabolism [10].
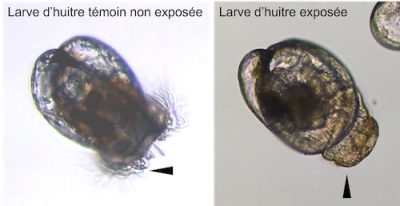
- Finally, heavy metals, PAHs or even some drugs have the capacity to alter the physiology of the oyster; the effects of these molecules being closely linked to their mechanism of action. For example, it has been demonstrated that antidepressants have the capacity to disrupt the embryonic development of oysters (embryotoxicity) until the metamorphosis of the larvae.
The validity of these laboratory results must now be evaluated in the more complex conditions of the natural environment. Work carried out on organisms taken directly from the natural environment makes it possible to establish the link between environmental contamination, the bioaccumulation of pollutants in animal’s tissues and the state of health of the organisms. An Indian study, carried out on several sites of the Arabian Sea coast, characterized by a contamination by PAHs and heavy metals, highlighted a correlation between the bioaccumulation of various PAHs and the damage to the DNA integrity in Saccostrea cucullata oysters; damage whose capacity to disturb the progeny of exposed organisms in oysters is now known [11]. These results raise the question of the repercussion of the individual effects observed on populations and, more broadly, on ecosystems: a field of research that remains largely unexplored to this day…
2.3. New particulate contaminants: microplastics

On average, 80% of plastic debris found at sea comes from land (wind, rivers, runoff, wastewater) and the rest from maritime activities (tourism, boating, fishing, aquaculture). The impacts of plastics at sea can be presented in two main areas.
Firstly, they promote the transport of species. Once at sea, plastics constitute a habitat favourable to the colonisation of micro-organisms (bacteria, fungi, protozoa, viruses) called the “plastisphere”. Some of these species are toxic or pathogen, such as families of bacteria known to be pathogen to oysters. The question then arises as to whether these pathogen species disseminated on the surface of plastics can transmit diseases?
The second major category of impacts of plastic waste at sea is only visible on large marine animals (birds, mammals or turtles) trapped in plastic waste or obstructing their respiratory or digestive tracts. However, this is only the tip of the iceberg as more than 90% of plastic waste floating at sea is smaller than 5 mm, known as microplastics (Figure 5). Because of their small size, these microplastics are easily ingested through the food chain. The first to be exposed are filter feeders, including oysters.
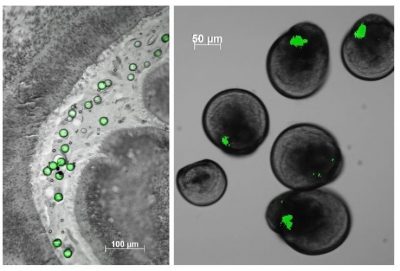
As for the smallest ones, the nanoplastics, whose quantities in Ocean are still unknown due to the lack of innovative methods, their small size allows them to interact with the biological membranes. Since oysters fertilize externally, their gametes, once released into the sea, are subject to environmental hazards. While polystyrene microbeads have no effect (under our experimental conditions) on oyster gametes, embryos and larvae, the 50 nanometer nanobeads strongly disturb them. How do you tell me?
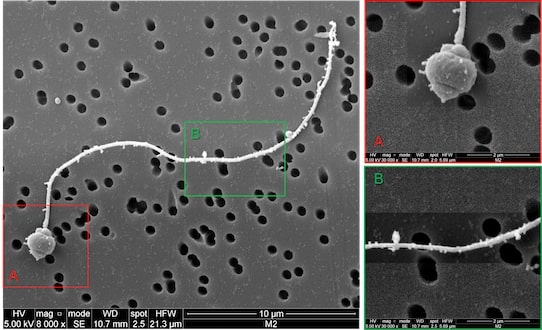
- Nanoplastics have been observed by electron microscopy stuck to the surface of oyster spermatozoa (Figure 7), which would prevent their mobility necessary for their fertilizing ability [13].
- Exposed for 24 hours to these nanobeads, oyster embryos show malformations up to complete developmental arrest at high doses.
Finally, the diet of oysters, and more broadly of bivalves, may also be impacted since the life cycle of diatom micro-algae also appeared to be disrupted by these nanoplastics, showing the importance of understanding the effects of micro- and nano-plastics at the complex scale of ecosystems.
3. What can be done? Are there any solutions?
Many solutions exist or are to be discovered to decrease or stop the main anthropogenic pressures. We can cite the example of tributyltin, which was banned in France in 1982 and then generalized worldwide in 2001 due to its proven toxicity and environmental risks. However, finding alternatives to tributyltin, which are non-toxic and therefore without risk to the environment, is a complex issue requiring research and technological innovation. And this reasoning becomes more complex when considering the diversity of pollution, its sources and uses. No single solution can solve the problems.
Scientific monitoring and research are necessary to understand pollution and its effects in nature, and just as much to raise awareness among the general public and decision-makers. They are notably framed at the European level in the Marine Strategy Framework Directive (MSFD) [14], the objective of which is to achieve a “good ecological status” of European marine waters by 2020, a goal that will be extended in the years to come.
A juxtaposition of solutions along the entire value chain is and will therefore be necessary to limit and stem pollution at source. This involves :
- Sobriety from the extraction of raw materials and production to their use (for example, for plastics, see the French parliamentary report [15]), and therefore changes in our industrial, agricultural and citizen behaviour;
- A much better management of the end-of-life of products and waste;
- Technological developments in industry, especially for new processes, products, materials such as bio-sourced and/or biodegradable, in any case without impacting the environment and human health (concept of safe-by-desing);
- Legislation, in particular environmental law, for the development of legal rules aimed at protecting the environment. Protecting our coastal marine areas, so precious, is a way to strengthen the integrity of the ocean system, a common good of humanity.
4. Messages to remember
- Many pollutants, pesticides, drug residues, heavy metals, microplastics, have effects on the entire life cycle of the oyster.
- Some toxic effects can be propagated to the next generation of young oysters (embryotoxicity, endocrine disruption).
- The “cocktail” effect of these pollutants (and more broadly the exposome) remains to be studied for the understanding of the evolution of oyster populations.
- Oysters, present on our coasts for millions of years, are now alerting us to the need for strong measures to protect coastal habitats.
Notes and references
Cover image. Oyster larvae that ingested green microplastics during a laboratory experiment [Source: © IFREMER / Rossana Sussarellu]
[1] Thomas, Y. (2018). Oysters as sentinels of climate variability and climate change in coastal ecosystems. Environmental Research Letters 13, 104009. Publisher’s official version : https://doi.org/10.1088/1748-9326/aae254, Open Access version : https://archimer.ifremer.fr/doc/00461/57255/
[2] https://perturbateursendocrinienssite.wordpress.com/2017/05/04/tributyletain/
[3] https://fr.wikipedia.org/wiki/Antifouling
[4] Chapelle, A. (2016). Modélisation du phytoplancton dans les écosystèmes côtiers. Application à l’eutrophisation et aux proliférations d’algues toxiques. https://archimer.ifremer.fr/doc/00360/47141/
[5] https://wwz.ifremer.fr/envlit/Surveillance-du-littoral/Phytoplancton-et-phycotoxines
[6] Payton, L. (2017). Chronobiologie moléculaire et comportementale des huîtres Crassostrea gigas diploïdes et triploïdes exposées à l’algue toxique Alexandrium minutum. Thèse de l’Université de Bordeaux. https://tel.archives-ouvertes.fr/tel-01579783/document
[7] Castrec, J (2018). Impacts des efflorescences du dinoflagellé toxique Alexandrium minutum sur la reproduction et le développement de l’huître Crassostrea gigas. Thèse de l’Université de Bretagne occidentale. https://tel.archives-ouvertes.fr/tel-03035012/document
[8] Gadelha, J.R. (2019). Persistent and emerging pollutants assessment on aquaculture oysters (Crassostrea gigas) from NW Portuguese coast. Science of the Total Environment, 666, 731-742.
[9] Bachere, E. (2017). Parental diuron-exposure alters offspring transcriptome and fitness in Pacific oyster Crassostrea gigas. Ecotoxicology and Environmental Safety, 142, 51-58. https://doi.org/10.1016/j.ecoenv.2017.03.030
[10] Epelboin, Y. (2015). Energy and Antioxidant Responses of Pacific Oyster Exposed to Trace Levels of Pesticides. Chemical Research In Toxicology, 28, 1831-1841. Publisher’s official version : https://doi.org/10.1021/acs.chemrestox.5b00269, Open Access version : https://archimer.ifremer.fr/doc/00284/39490/
[11] Melwani, A.R. (2016). PAHs and heavy metals at each sampling location along the west coast of India around Goa, India. Aquatic Toxicology, 173, 53-62.
[12] Sussarellu, R. (2016). Oyster reproduction is affected by exposure to polystyrene microplastics. Proc. Natl. Acad. Sci. USA, 113, 2430-2435. Open Access version: https: //archimer.ifremer.fr/doc/00311/42233/
[13] Tallec, K. (2019). Impacts des nanoplastiques et microplastiques sur les premiers stades de vie (gamètes, embryons, larves) de l’huître creuse Crassostrea gigas. Thèse de l’Université de Bretagne occidentale. https://tel.archives-ouvertes.fr/tel-02996550/document
[14] https://wwz.ifremer.fr/Expertise/Eau-Biodiversite/Directive-Cadre-Strategie-pour-le-Milieu-Marin
[15] http://www.senat.fr/fileadmin/Fichiers/Images/redaction_multimedia/2020/2020-Documents_pdf/20201512_Conf_presse_OPECST/Rapport_final.pdf
The Encyclopedia of the Environment by the Association des Encyclopédies de l'Environnement et de l'Énergie (www.a3e.fr), contractually linked to the University of Grenoble Alpes and Grenoble INP, and sponsored by the French Academy of Sciences.
To cite this article: HUVET Arnaud, SANCHEZ Wilfried, POUVREAU Stéphane, FABIOUX Caroline (January 5, 2025), The oyster, the sentinel of a coastline to be preserved, Encyclopedia of the Environment, Accessed January 22, 2025 [online ISSN 2555-0950] url : https://www.encyclopedie-environnement.org/en/life/oyster-sentinel-of-coastline-to-be-preserved/.
The articles in the Encyclopedia of the Environment are made available under the terms of the Creative Commons BY-NC-SA license, which authorizes reproduction subject to: citing the source, not making commercial use of them, sharing identical initial conditions, reproducing at each reuse or distribution the mention of this Creative Commons BY-NC-SA license.







