The root system of plants: from the shadows to the light
PDF
Roots are a prime target for crop improvement towards a more frugal and environmentally friendly agriculture. However, root traits have long been neglected in breeding programs, especially because the first green revolution was based on massive use of water and fertilizers. Global changes are now encouraging a more rational use of agricultural inputs in order to reduce soil degradation, water and air pollution, and resource depletion. Meeting production needs while limiting the use of water and mineral elements is a major challenge. What role can roots, the plant’s nourishing organs, play in the development of a more sustainable and resilient agriculture in the face of current and future climates? An essential role, as shown by recent research that allows us to better understand root system development, functions, diversity and adaptations to the environment, bringing roots from the shadows to the light.
1. Roots capture water and nutrients for the plant
Roots are organs of higher plants that are specialized in exploring the soil and taking up water and mineral ions (See How to feed plants while polluting less?). They play an essential role in plant survival and development. Each plant has several categories of roots organized in a root system. This set of roots anchors the plant in the soil and communicates with the stem(s), which carry the leaves, flowers and fruits. The root system develops through the repeated formation of new roots and their growth in length. Roots form and grow in tight relation to the plant’s environment. In doing so, roots allow plant adaptation to the heterogeneous and variable soil resources and constraints of its living environment (See The fixed life of plants and its constraints, The tireless quest for water by plants & Water needs of plants: how to satisfy them?).
1.1. Architecture and anatomy of the root system
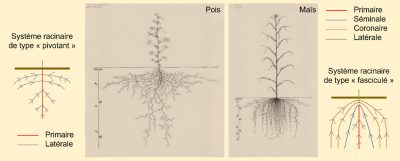
However, two main types of root systems can be distinguished [1]. The “taproot” system, found in dicotyledonous Angiosperms (such as rapeseed or pea) and Gymnosperms (such as pine), is most often characterized by the formation of lateral roots on a main seminal root that grows in the soil like a pivot (Figure 1a) [2]. The majority of monocotyledonous Angiosperms (such as wheat or rice) have a “fibrous” root system characterized by several seminal roots and numerous so-called crown roots that are relatively equivalent in size and form from the base of the stems (Figure 1b). These roots themselves carry lateral roots. Some plants with a “fibrous” root system can develop more than a hundred crown roots.
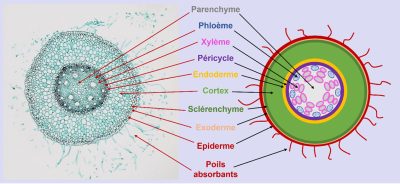
The specific properties of these different root tissues allow the root to modulate its exchange capacities with the soil:
- In particular, the root hairs drastically increase the exchange surface of the root and facilitate the acquisition of water and nutrients.
- More or less impermeable barriers surrounding the cells in the outer cortex and the endodermis regulate the flow of water and nutrients to the conducting vessels [3].
The vascular tissues channel sap exchanges between the roots and the aerial organs:
- The xylem vessels contain the raw sap composed of water and mineral elements that circulates from the roots to the aerial organs.
- The phloem vessels contain the elaborated sap rich in photosynthesis products that circulates from the aerial organs to the roots.
In perennial species, old roots enlarge over the years by adding new layers of tissues. These so-called secondary tissues form, among other things, cork in the periphery and wood in the center of the root.
1.2. Development and plasticity of the root system
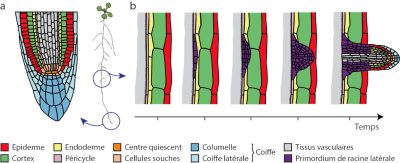
Root development in the soil is very sensitive to the environment. Root growth, development, and functional properties are influenced by signals circulating within the plant and stimuli perceived from the soil. These modifications of the root system in response to changing environmental conditions are crucial for plant adaptation and are referred to as root plasticity.
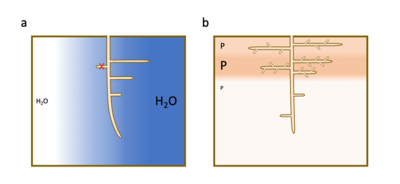
- auxin accumulates preferentially in the zones of the root in contact with the humid soil and stimulates the formation of a new root meristem.
- abscisic acid, a hormone involved in plant responses to water stress, accumulates preferentially in tissues in front of dry soil regions and suppresses the formation of lateral roots.
As with water, a high local accumulation of phosphorus and nitrate in poor soils induces a preferential proliferation of lateral roots in these particular areas [7] (Figure 4b).
Thus, the plant root system is a complex structure that develops continuously in the soil and adapts dynamically to it. Adaptive root plasticity is favorable for plant nutrition under resource-limited conditions. Moreover, through their activity, these roots interact with the soil and modify its characteristics.
2. Roots interact with the soil and its microbial communities
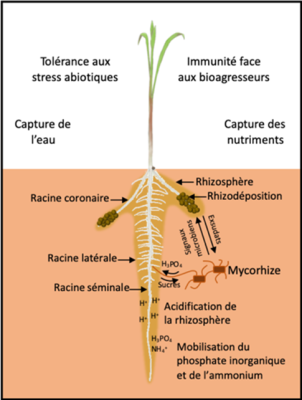
2.1. Influence of roots on soil characteristics
Some nutrients are present in the soil in a chemical form inaccessible to the plant. Root exudation contributes to make these elements more available to the roots. Mechanisms of soil acidification by the exudation of protons or phytosiderophores [9] promote the acquisition of iron in certain plants [10]. Root exudation is also a plastic process and depends on the environment in which the root develops. White lupin plants growing in phosphorus-poor soil, for example, produce short and dense root clusters called “proteoid roots” [11]. These roots secrete large amounts of acidic molecules capable of lowering the soil pH, making phosphorus ions more assimilable. In this plant species adapted to nutrient-poor soils, this plasticity of root activity is a major adaptation mechanism. In one experiment, it was shown that a lupin plant growing on phosphorus-poor soil can secrete up to 25 times more exudates than a plant growing on phosphorus-rich soil [12].
Exudation also contributes to the formation of a rhizosheath . This rhizosheath corresponds to the aggregation, around the roots, of a soil rich in mucilage and other carbonaceous molecules, which facilitates water acquisition by the plant. In chickpea, a large rhizosheath has been associated with a better capacity of the plant to tolerate water stress [13].This aggregation of particles around the roots could increase soil moisture in contact with the root epidermis and help maintain the contact and exchange zone between the soil and the root under drought conditions. In addition, this aggregated soil rich in exudates represents an important source of carbon for microorganisms potentially able to establish beneficial interactions with the plant.
2.2. Mobilization of microorganisms by the roots
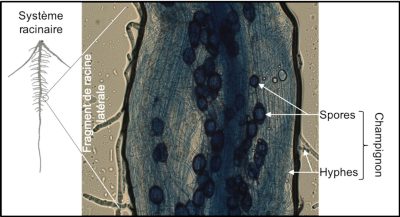
The rhizosphere is generally microbiologically very active. Some root exudates act as attractive signals for microorganisms [14]. Strigolactone molecules [15] secreted by the plant into the rhizosphere stimulate the germination of spores of arbuscular mycorrhizal fungi. The molecules produced by the fungus in turn promote the initiation of root processes that allow the establishment of a symbiosis between the plant and the fungus (Figure 6). During this symbiosis, the exploration of a very large volume of soil by the mycelial hyphae of the fungus contributes to the supply of water, phosphorus and other mineral elements to the plant. In return, the fungus benefits from the carbon molecules produced by plant photosynthesis.

By their continuous and plastic development, their adaptable properties and their beneficial actions on the rhizosphere, roots promote the nutrition and health of the plant. Improving the characteristics of the root system of cultivated plants allows to optimize these properties in increasingly constrained agronomic contexts.
3. The root system: a target for plant improvement
3.1. Diversity and complexity of root systems
The root development of a single plant develops and adapts dynamically to environmental conditions. A great diversity in the way root systems develop and adapt to different environments can be observed between individuals of the same plant species. This diversity of root systems between varieties of the same species is the result of a progressive selection, over the course of evolution, of certain genetically controlled adaptive traits.
In rice, distinct root architectural traits have been identified in varieties adapted to different growing systems:
- Varieties adapted to irrigated cultivation (where the field is permanently covered with water) have a root system that tends to colonize the superficial layers of the soil;
- Varieties adapted to rainfed cultivation (where the water supply depends only on intermittent rainfall) develop a deeper root system.

This diversity of root traits between plants of the same species can be exploited for varietal improvement. The transfer of beneficial root traits from one variety to another by crossing is an improvement strategy.
Following this principle, Japanese researchers have been able to improve the drought tolerance of the rice variety IR64. This variety is very productive and its grains have desirable quality traits. Nevertheless, its shallow root system does not allow the plant to take up enough water when the first few centimeters of soil dry out. In contrast, another rice variety, called Kinandang Patong, is less productive but better adapted to drought thanks to the ability of its root system to capture water from deep soil layers. A cross between these two varieties was conducted to introduce the “deep root system” trait in IR64 [18]. A new rice variety combining the agronomic characteristics of IR64 with the deep root system and drought tolerance of Kinandang Patong was then created.
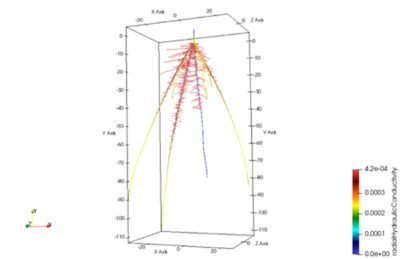
In silico models of root systems interacting with soil models have been developed and allow simulating root system structure and functions as a whole (Figure 9). These models facilitate the identification of root traits that will, ultimately, show beneficial effects on plant nutrition and development in a given environment.
3.2. Utility of root systems for plant breeding
Powerful methods are now available to visualize and describe quantitatively the characteristics of the root system of plants without disturbing them. Nevertheless, these data are generally collected using laboratory experimental set-ups that poorly reflect the conditions of plants grown in the field. To meet the need of characterizing plant root systems under agronomic conditions and to facilitate their consideration in plant breeding programs, several methods for studying root systems in field have been developed.

By increasing the number of observations and collected data, the development of these new methods of root exploration in the field are decisive to:
- characterize the diversity of root traits and their plasticity
- and identify the regions of the genome involved in the control of these traits.
4. Take home messages
- Root architectural and anatomical traits influence the ability of plants to acquire water and nutrients and to adapt to certain stresses including drought.
- The development and functioning of the root system respond dynamically to the environment. This phenomenon called root plasticity strongly contributes to the adaptation capacities of the plant to environmental constraints.
- The exudation of carbon compounds by the roots in the soil allows to modify the physical, chemical and biological characteristics of the rhizosphere. It facilitates the acquisition of water and nutrients and is involved in the establishment of symbiotic interactions with soil microorganisms.
- Not all plants have the same root system. There is great diversity, between species and between plants of the same species in the way roots develop, function, adapt to the environment and interact with the soil. This diversity, linked to differences in genetic heritage between plants, can be exploited in plant breeding.
- In research efforts targeting plant root performance, the whole root system and its complex relationships with its environment need to be considered. For this purpose, efficient characterization methods in the field and modelling strategies are useful.
- Selection of crop varieties with favourable root traits has improved the performance of several species of agronomic interest, such as wheat, maize, and soybean. This research strategy contributes to a more sustainable agriculture, aiming to improve the food security of populations.
References
Cover image. Tree Roots (1890), last painting by Van Gogh (1853-1890), Van Gogh Museum, Amsterdam. [Source: Vincent van Gogh, Public domain, via Wikimedia Commons]
[1] Atkinson JA, Rasmussen A, Traini R, Voss U, Sturrock C, Mooney SJ, Wells DM, Bennett MJ. 2014. Branching out in Roots: Uncovering Form, Function, and Regulation. Plant Physiology 166, 538-550
[2] Original drawings of pea and maize root systems in Figure 1 are from: Kutschera, L.; Lichtenegger, E.; Sobotik, M., Würzelatlas der Kulturpflanzen gemässigter Gebiete mit Arten des Feldgemüsebaues. – Frankfurt am Main: DLG-Verlag, 2009 (2. Aufl. 2018). – 527 p. ; ; Wageningen University & Research Image Collections, Root systems drawings; website: https://images.wur.nl/digital/collection/coll13/search
[3] Doblas VG, Geldner N, Barberon M. 2017. The endodermis, a tightly controlled barrier for nutrients. Current Opinion in Plant Biology 39, 136-143
[4] Trinh CD, Laplaze L, Guyomarc’h S. 2018. Lateral root formation: building a meristem De Novo. Annual Plant Reviews Online 1, 847-890
[5] Auxin provides cells with positional information enabling the changes in cell identity regulating the dynamics of morphogenesis in plants. Cells can read the concentration of auxin not only in space but also in time. https://www.insb.cnrs.fr/fr/cnrsinfo/lauxine-permet-aux-cellules-de-lire-lespace-et-le-temps
[6] Orman-Ligeza B, Morris EC, Parizot B, et al. 2018. The Xerobranching Response Represses Lateral Root Formation When Roots Are Not in Contact with Water. Current Biology 28, 3165-3173.e5
[7] Motte H, Vanneste S, Beeckman T. 2019. Molecular and Environmental Regulation of Root Development. Annual Review of Plant Biology 12, 1-24
[8] de la Fuente Cantó C, Simonin M, King E, Moulin L, Bennett MJ, Castrillo G, Laplaze L. 2020. An extended root phenotype: the rhizosphere, its formation and impacts on plant fitness. Plant Journal 103, 951-964
[9] Phytosiderophores are ligand-like molecules (or chelators) secreted into the rhizosphere by certain plant species of the Poaceae family (grasses) in iron deficiency situations, whose function is to allow iron uptake.
[10] Chen YT, Wang Y, Yeh KC. 2017. Role of root exudates in metal acquisition and tolerance. Current Opinion in Plant Biology 39, 66-72
[11] Proteoid roots are plant roots that form dense clusters of short, closely spaced lateral rootlets. They can form a thick mat two to five centimeters thick just below the leaf litter. They provide intense uptake of nutrients from the soil, probably by chemically altering their environment in the soil to enhance nutrient solubilization.
[12] Johnson JF, Allan DL, Vance CP, Weiblen G. 1996. Root carbon dioxide fixation by phosphorus-deficient Lupinus albus. Contribution to organic acid exudation by proteoid roots. Plant Physiology 112, 19-30
[13] Rabbi SMF, Tighe MK, Flavel RJ, Kaiser BN, Guppy CN, Zhang X, Young IM. 2018. Plant roots redesign the rhizosphere to alter the three-dimensional physical architecture and water dynamics. New Phytologist 219, 542-550
[14] Venturi V, Keel C. 2016. Signaling in the Rhizosphere. Trends in Plant Science. 11, 4265-4281
[15] Carotenoid-derived phytohormones, strigolactones control plant branching, drive seed germination of parasitic phanerogams, and stimulate the growth of symbiotic mycorrhizal fungi.
[16] Ramírez-Flores MR, Perez-Limon S, Li M, Barrales-Gamez B, Albinsky D, Paszkowski U, Olalde-Portugal V, Sawers RJH. 2020. The genetic architecture of host response reveals the importance of arbuscular mycorrhizae to maize cultivation. eLife 9, 1-18
[17] Schneider HM, Klein SP, Hanlon MT, Kaeppler S, Brown KM, Lynch JP. 2020. Genetic control of root anatomical plasticity in maize. Plant Genome 13, 1-14
[18] Uga Y, Sugimoto K, Ogawa S, et al. 2013. Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nature genetics 45, 1097-102
[19] Developed by Prof. Jonathan Lynch, this method allows to visually record architectural features of a plant’s root crown in the field within minutes. The term “shovelomics” is derived from the words “shovel” and “omics” (a method for generating massive data and, more importantly, for analyzing and extracting the knowledge necessary for a better understanding of living things).
[20] Atkinson JA, Pound MP, Bennett MJ, Wells DM. 2019. Uncovering the hidden half of plants using new advances in root phenotyping. Current Opinion in Biotechnology 55, 1-8
The Encyclopedia of the Environment by the Association des Encyclopédies de l'Environnement et de l'Énergie (www.a3e.fr), contractually linked to the University of Grenoble Alpes and Grenoble INP, and sponsored by the French Academy of Sciences.
To cite this article: GRONDIN Alexandre, GUYOMARC'H Soazig, LAPLAZE Laurent (January 5, 2025), The root system of plants: from the shadows to the light, Encyclopedia of the Environment, Accessed April 21, 2025 [online ISSN 2555-0950] url : https://www.encyclopedie-environnement.org/en/life/root-system-plants/.
The articles in the Encyclopedia of the Environment are made available under the terms of the Creative Commons BY-NC-SA license, which authorizes reproduction subject to: citing the source, not making commercial use of them, sharing identical initial conditions, reproducing at each reuse or distribution the mention of this Creative Commons BY-NC-SA license.







