The biosphere, a major geological player
PDF
The biosphere, that is, the terrestrial environment and the living organisms that have developed there, deeply shapes the planet Earth. With the genesis of carbonaceous rocks, the biosphere is co-responsible for the presence of atmospheric oxygen and its variations. It is a major player in the manufacture of limestone and other sedimentary rocks. Thanks to limestones and, to some extent, carbonaceous rocks, the biosphere participates in the long-term variations of atmospheric CO2. And even the continental biosphere is a major player in the chemistry of the oceans and the rocks that are formed there.
1. The facts of the problem
In recent years, there has been rightly concern about the negative impact of civilization on the environment. If humanity is fortunately unable to destroy the planet, it can significantly disturb its upper envelopes: atmosphere, hydrosphere, lithosphere and biosphere. The different actors of the biosphere have always interacted with each other (thus, lions eat antelopes which, if they were in excess, would devastate entire ecosystems…). But, independently and before the disruptive actions of a single species (Homo sapiens) that seriously began only with the Neolithic revolution, did other species influence the Earth’s envelopes? Has life influenced, or even drastically changed, the atmosphere, the hydrosphere, the earth’s crust…?
2. Life and atmospheric oxygen
2.1. Ecosystems, through photosynthesis, produce oxygen
Green plants, algae and many bacteria make photosynthesis : they capture water (H2O) and carbon dioxide (CO2) and transform them into carbohydrates and oxygen (O2) using sunlight energy. The photosynthesis equation can be summarized as follows:
6 CO2 + 12 H2O → C6H12O6 (glucose) + 6 H2O + 6 O2
equation that can be simplified and remain accurate in terms of mass balance (while being false from the point of view of reaction mechanisms): CO2 → C (Carbon) + O2.
Considering the respective atomic masses (C = 12 g, O = 16 g), it can be said that 44 g of CO2 gives 32 g of O2 and 12 g of C fixed in the biomass (proportion of 32/12) by photosynthesis. Is this release of O2 by current and recent photosynthesis alone sufficient to explain all atmospheric O2?
2.2. Ecosystem respiration consumes the oxygen produced by photosynthesis

But my mom forgot that trees, planktonic organisms… are deadly. When an oak dies, it is preyed upon by insects xylophages, moulds… that “feed” on lignin. After a few years, all the wood will have been decomposed by breathing organisms. This breathing will absorb 26 tons of O2 that will oxidize the 10 tons of carbon in the wood, producing 36 tons of CO2. We have returned to the starting point, and the balance is totally zero: no CO2 absorbed, no O2 produced! All balanced ecosystems, from Amazonian forests to ocean phytoplankton, have a zero balance against O2 and atmospheric CO2. Of course, a young growing forest growing on soil previously devoid of vegetation or a planktonic bloom temporarily produces O2. But as soon as the forest matures and each growing tree “replaces” a recently dead tree, as soon as theplanktonic bloom stops, the balance is zero.
There is about 3,000 x 1012 kg of reduced carbon in the biosphere, taking into account all living biomass or, very temporarily, stored in the soil (see A carbon cycle disrupted by human activities). The production of this current biomass has produced O2 in the mass proportions of 32/12, or 8,000 x 1012 kg of O2. However, the current atmosphere contains about 1,000,000 x 1012 kg, or 125 times more. More than 99% of the current atmospheric O2 is not the counterpart of the current biomass. A forest would be like a pastry cook who bakes cakes but eats them before selling them; he is not the one who supplies the city!
So where does this current oxygen come from, which comes neither from forests nor from living plankton…?
2.3. Where does today’s atmospheric oxygen come from, since current forests and plankton are not enough?
For a photosynthetic ecosystem (forest, phytoplankton, etc.) to produce O2 in the very long term, a process must prevent dead organic matter from being consumed and re-oxidized in this very long term. This process is geological, it is the fossilization of organic matter. When, after their growth, geological circumstances cause dead trees, soils or plankton to settle and become coal, oil or fossil organic matter dispersed in clays or marls, then there is a net input of O2 into the atmosphere. Each time 12 g of reduced carbon is produced and fossilized, the 32 g of O2 produced by photosynthesis remains in the atmosphere because it is not used by breathing organisms. The current 1,000,000 x 1012 kg of atmospheric O2 is, in mass, the fossil reduced carbon counterpart of sedimentary rocks (coal, oil…), and not of the Amazonian forest or living plankton.

2.4. Iron oxides
Current seawater contains almost no iron, as it is oxidized by dissolved oxygen in the form of ferric ion (Fe4+), insoluble at ordinary pH. Recent iron-bearing sedimentary rocks are therefore rare, due to the lack of soluble iron to precipitate. However, the situation was completely different before -2 billion years ago.
From -3.8 billion years ago, the age of the oldest sedimentary rocks, to -2.5 billion years ago, iron ores in the Fe4+ form are present, but dispersed and in relatively limited quantities. This presence of Fe4+ shows that there were oxidizing environments at least locally, so that very probably there was an oxygenic photosynthesis very early in the history of the Earth. Stromatolites (see Figure 6 and introductory photo of the article) aged 3.5 billion years also suggest this precocity. These iron-rich Fe4+ ores explode in quantity around -2.5 billion years, then almost disappear around -2 billion years. They consist of alternating silica and iron oxides Fe4+, hematite (Fe2O3) most of the time. They are called “Banded Iron Formation” (BIF). Where do they come from? Before -2.5 billion years ago, the atmosphere and ocean contained almost no O2: 10-6 compared to the current amount. The sea contained iron in the ferrous state Fe2+, soluble, brought by the hyperactive volcanism at that time. The combination of “photosynthesis and fossilization of organic matter” produced O2, but this O2 oxidized Fe2+ iron, which precipitated as banded Fe3+ iron (Figure 3). Oxygen did not accumulate in the atmosphere or the ocean. Around -2.5 billion years ago, due to a combination of complex and still poorly understood reasons in 2016 (biological and metabolic revolutions perhaps, major changes in volcanism and tectonics certainly, climatic variations undoubtedly…), O2 is multiplied by 100 000 (Figure 4). All the Fe2+ of the sea precipitates, and forms the gigantic deposits of Fe3+ dated -2.5 billion years ago (see Figure 2). This event is known as Great Oxygenation (GO) (see Focus Oxygen, a revolution). This Great Oxygenation was certainly accompanied by a major biological crisis since O2 must have been very toxic to organisms of the time, mainly anaerobic; but the fossil record is far too incomplete to appreciate its importance.

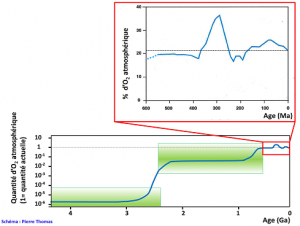
Since this Great Oxygenation, there is almost no more Fe2+ to precipitate into the sea. Oxygen varies very slowly, but generally increases more often than it decreases, due to relative variations between photosynthesis and trapping of organic matter in sediments that produce O2 and other phenomena that consume it such as oxidation of iron of magmatic origin, mineral sulphur, old sedimentary rocks, etc. A content of more than 15% seems to have been reached for 600 million years. Since then, this content has fluctuated between 15 and 35% depending on the relative importance of production and consumption in the long term, a relative importance regulated by interactions between biology and geology.

3. Life, rocks and CO2
3.1. The limestones
All you have to do is walking in the Alps, Quercy… to see a lot of limestone (CaCO3). In current nature, and this has most likely been the case for hundreds of millions of years, almost all limestone is of biological origin (Figures 5 to 8):
- Directly, when limestones consist of the accumulation of tests and shells of organisms that collect calcium and hydrogen carbonate ions from water (coccolithophorids, foraminifera, corals, bivalves, crinoids, etc.);
- Indirectly, when the presence of living organisms locally modifies environmental conditions and causes CaCO3 to precipitate (stromatolite, Figure 7).

Figure 6. Example of ancient limestone of coral origin: the limestones of the Upper Jurassic (150 Ma) of the southern Paris basin. [Source : © Photos Pierre Thomas]
The biosphere thus plays a massive role in the production of rocks on the surface of the Earth’s crust. This precipitation would be lifeless because surface seawater is saturated with calcium carbonate, but life catalyses, accelerates and localizes this precipitation. This precipitation is possible because there is a balance between dissolved CO2, HCO3–, CO32-, H+, Ca2+, solid CaCO3, etc. In the same way, on land, there is a balance between flush CaCO3, rainwater, CO2 from the atmosphere and the ground. These very complex balances can be summarized with a single formula:
2 HCO3– + Ca3+ ↔ CaCO3 + H2O + CO2 (1)
In the aquatic environment, the reaction goes mainly to the right (limestone precipitation), either by direct metabolism of living organisms that produce their test, or by capture of CO2 by photosynthesis of phytoplankton that shifts the balance to the right, or by mucoproteins bacterial matrices that catalyse carbonate precipitation…In the air, the reaction to the left takes place: dissolution of limestones in karsts (Figure 8). There is an overall balance in the medium term. The quantities of limestone and HCO3– in the sea, and CO2 in the sea and atmosphere, would be stable if no other phenomena were to occur.


However, another series of reactions can permanently modify the relative quantities of limestone, atmospheric CO2 and dissolved CO2: the alteration of rocks containing calcium silicates (very frequent case). This sequence of reactions can be summarized and schematized as follows:
(1) CaSiO3 (calcium silicate) + H2O + 2 CO2 → SiO2 (dissolved silica) + Ca3+ + 2 HCO3–
This reaction occurs in continental soils. Life participates in this stage because the soil is enriched with CO2 by the respiration of roots, fungi, soil bacteria (Figure 9). This CO2 comes from the atmosphere through plant photosynthesis. The ions involved are transported to the sea by runoff and rivers.

2 HCO3– + Ca3+ → CaCO3 + H2O + 1 CO2
As we have seen above, life has a lot to do with this reaction.
The assessment of these steps can be written as follows:
CaSiO3 + H2O + 2 CO2 → SiO2 + Ca3+ + 2 HCO3– → SiO2 + CaCO3 + H2O + 1 CO2 (3)
If the dissolution-precipitation of carbonates (equation 1) does not change the overall amounts of limestone or atmospheric CO2, the alteration of calcium silicates and the subsequent alteration (equation 3) increases the amount of limestone and decreases the amount of atmospheric CO2. Life actively participating in the alteration of silicates therefore actively participates in this mechanism of atmospheric CO2 reduction, much more than through the photosynthesis-fossilization of organic matter: there is more limestone than coal!

3.2. Siliceous rocks
The alteration of continental silicate rocks, favoured by life, releases silica which is brought to the sea by rivers. In the sea, organisms live with test or spicules silica (diatomaceous, radiolar, spongial). The accumulation of these tests and spicules can constitute huge sedimentary accumulations and form diatomites, radiolarites, gaizes (Figure 10).
4. Life on the continents, marine life, climate variations, water chemistry, sedimentary rocks: everything is linked!
4.1. Continental life, alteration/erosion and marine sedimentary rocks
Life on the continents favours the alteration of rocks. Roots increase rock fracturing and increase the surface area of rock-soil water exchange. Roots, fungi, bacteria produce respiratory CO2, organic acids that alter rock minerals much more than just rainwater (Figure 11). This alteration in soils produces ions that are exported by surface or groundwater, but also clays and unaltered minerals that are much less mobile than ions. On the other hand, the vegetation cover (grasses, tree roots) retains soil particles (clays, residual minerals) and limits erosion (Figure 12). The biosphere therefore promotes the chemical alteration of emerged rocks and the transfer of ions from the continent to the sea, but limits erosion and the transfer of solid particles to the sea.


Sedimentary rocks, to simplify, are of two types: rocks of (bio)chemical origin, especially limestones, formed by the precipitation of ions dissolved in water, and detrital rocks, formed by the sedimentation of solid particles (mud, sand, gravel, pebbles…) brought to the sea by rivers. The continental biosphere, which promotes the release of ions but limits erosion and the release of detrital particles, has a significant influence on marine sedimentation. The evolution of the continental biosphere over the geological ages has completely changed the nature of the sea and marine sediments.
4.2. Biosphere-planet Earth interactions over the last 542 million years
It was in the Devonian (-420 to -360 million years ago) that Evolution “invented and selected” lignin and trees. Before the Lower Devonian (-420 million years ago), continental vegetation was very reduced. At the end of the Upper Devonian (-360 million years), most of the continents were to be covered by forests, which then prospered considerably in the Carboniferous (-360 to -300 million years), the period following the Devonian (Figure 13, [1]).
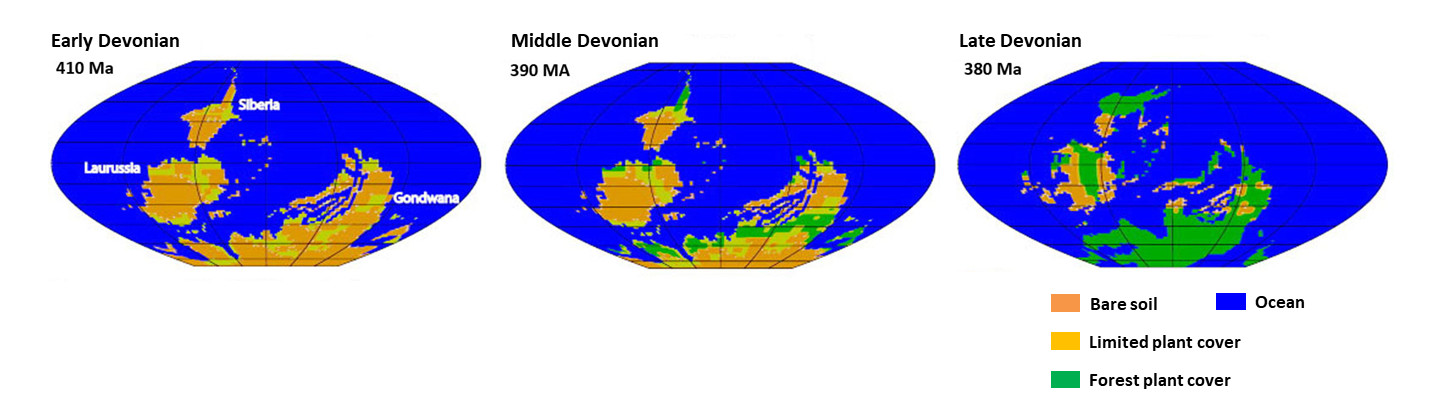
The history of the Phanerozoic (Figure 14) is punctuated by five major extinctions, where at least 50% of fossil biodiversity disappears in a very short geological time. One of these extinctions, the second chronologically speaking, takes place in the Terminal Devonian (around -374 million years ago). Like the other four, this extinction is probably multifactorial, but one of the proven causes is an anoxia widespread oceanic. This ocean anoxia is believed to be due to two causes directly related to the colonization of continents by forests, which has completely disrupted all ecosystems and led to a temporary “eutrophication” from the seas:
(1) sudden inflows of organic matter from continental soils being installed;
(2) planktonic blooms due to the sudden richness of the sea in ions and other mineral nutrients;
(3) consumption of all O2 dissolved in water during the decomposition of dead bodies of accumulated plankton.
It is often said that the appearance of Man is responsible for the sixth extinction that is looming; but who knows that the appearance of trees is probably partly responsible for the second extinction? And all these major changes just because Evolution “invented and selected” lignin and supporting tissues!
5. Life, ocean stratification and the nature of ocean sedimentary rocks

As for the depth of the CCD, it is a function, among other things, of the release of deep CO2 which tends to raise it and the rain of carbonate tests (the corpses of planktonic organisms) which tends to lower it. Since Evolution “invented and selected” an abundant pelagic plankton with calcareous test (since the Jurassic, -201.3 to -145 million years ago), the CCD is several kilometres deep. Before the Jurassic, there was no calcareous plankton and no carbonate rain; the CCD was much more superficial. And there are no oceanic limestones before the Jurassic. Life and its variations are therefore major actors in ocean chemistry.
6. As conclusion and perspective
In everything that has just been said, important points have not been addressed, for example everything related to methane, phosphates, the sulphur cycle… And moreover, we have only talked about the superficial biosphere, which is only the tip of the iceberg. About twenty years ago, we discovered a whole biosphere made up of bacteria and archaea living in the first few kilometres of the lithosphere, both continental and oceanic: theendogenous life. These microorganisms can be heterotrophic and use the organic carbon present in these first few kilometres. They are often autotrophic (more precisely chemiolithotrophic) and live through reactions such as:
Fe2+ of silicates + H2O + CO2 → Fe4+ + organic molecules
Such a metabolism certainly modifies, but in what proportion, the chemistry of the crust (or even the upper mantle). All that remains is to study what is perhaps the main (mass) compartment of the biosphere.
Everything that has just been said concerns the action of the biosphere on the other envelopes of the planet (atmosphere, hydrosphere, crust…). That is why we cannot be a “complete” geologist if we are not also a bit of a biologist and ecologist. But the reverse is true. The Earth’s “mineral” envelopes influence the biosphere: the atmosphere through its climates and their variations, the hydrosphere through its movements and composition, the solid Earth through its chemistry, its slow movements (“continental drift” and its action on evolution), its violent crises (giant volcanic blooms called trapps…), have an influence on the biosphere. You can’t be a “complete” biologist and ecologist if you’re not also a bit of a geologist.
References and notes
[1] Le Hir G. et al. (2011) The climate change caused by the land plant invasion in the Devonian. Earth and Planetary Science Letters 08-042; http://dx.doi.org/10.1016/j.epsl.2011.08.042
The Encyclopedia of the Environment by the Association des Encyclopédies de l'Environnement et de l'Énergie (www.a3e.fr), contractually linked to the University of Grenoble Alpes and Grenoble INP, and sponsored by the French Academy of Sciences.
To cite this article: THOMAS Pierre (January 5, 2025), The biosphere, a major geological player, Encyclopedia of the Environment, Accessed March 25, 2025 [online ISSN 2555-0950] url : https://www.encyclopedie-environnement.org/en/life/the-biosphere-a-major-geological-player/.
The articles in the Encyclopedia of the Environment are made available under the terms of the Creative Commons BY-NC-SA license, which authorizes reproduction subject to: citing the source, not making commercial use of them, sharing identical initial conditions, reproducing at each reuse or distribution the mention of this Creative Commons BY-NC-SA license.




