氧气与生命之间的危险联系

氧气即生命!在如今任何环境下,许多生物过程如果没有氧气(O2)的参与就不可能发生,绝大数生物一旦离开了氧气就会死亡。但有些生物在没有氧气的情况下依然能够生存,甚至对某些生物来说,氧气是一种剧毒物质。那在过去呢? 实际上在过去近20亿年的时间里,地球上的生命是在没有氧气的环境下发展起来的,因为在地球的大气中或海洋中的水中很少或没有氧气(水是生命的必需品!),而氧分子是某些细菌如蓝藻细菌代谢的副产物。是生命创造了氧气!然后它必须设法在这种强烈的氧气环境中继续生存。本文讨论的正是生命与氧气之间的这种激烈的对抗关系。
在我们的认知里,氧气(氧分子)与生命密切相关。与水分子一样,在公众的眼中,氧气可能是表征生命的最重要的分子。没有氧气就没有生命。二者关系非常密切,亚马逊雨林遭到大面积砍伐而导致空气中氧气含量下降,甚至有可能演变成地球灾难。然而,生命与氧气之间并没有绝对的联系。曾经,地球上生命是在没有氧气的情况下生存和发展的,即使在今天,许多生物仍然在没有氧气的环境中生存,氧气对它们来说经常是一种致命的毒药。如果氧气在早期充斥着地球,那么地球很有可能永远不会出现生命。
1. 氧气是什么?
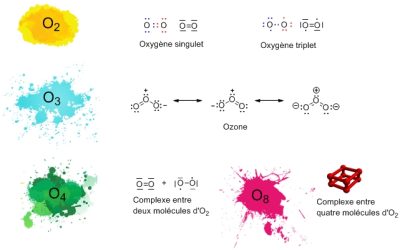
氧元素的原子序数为8(图1)[2],8个电子围绕其原子核旋转,其中的6个电子在任何给定的时间都能参与化学键的形成。两个氧原子之间通常形成两个键,每个键都带一个额外的电子。因此每个氧原子都被8个电子包围着,因此根据八隅规则,一个原子必须被8个结合电子包围着。氧气分子 O2 的形成过程就是这样。最简单的表示方法是在两个原子之间放置一个双键。但这是一种不准确的表述,在分子最稳定的形式中,每个氧原子保留一个“单”电子,这叫做三线态氧。带有双键的图(图 1)对应的是分子的另一种形式,称之为单线态氧,比三线态氧相比,其稳定性低得多并且更具反应性。
还有一些分子组成只有氧原子参与,例如臭氧(O3),它存在于地球上层大气中,由氧分子结合形成,它能有效吸收的一些太阳紫外线辐射保护人类免受其伤害。在实验室中还可以制备其他极其稀有的氧:O4 及固体氧中的O8。
2. 氧气的作用
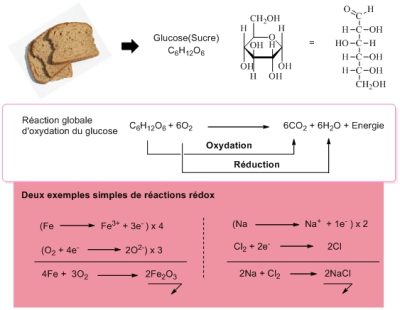
这几乎是不言而喻的,氧气是一种氧化剂。这就是它在生物学中的作用。例如它可以氧化我们摄入的糖(图 2),氧化产生能量随后为其他生命过程供能,如生物分子的合成等化学过程以及拉伸肌肉、行走、跑步、思考等物理过程。
氧化反应的机理是复杂的,往往与还原反应有关,我们说的是氧化还原过程[3]。在图2所表示的反应中,葡萄糖被氧化生成CO2和H2O,氧气被还原生成H2O。我们可以用电子传递来解释所有的反应。当分子接受额外的电子时将被还原,失去电子时被氧化。氧是电子受体,被还原生成水。在氧化还原反应链的末端(如呼吸链),氧捕获了所涉及的电子,成为最后的电子受体。
和所有动物和真菌一样,人类使用有机分子(例如糖)作为电子源和氧气受体:它们呼吸!植物也需要氧气和呼吸才能生存(即使它们能够通过光合作用产生氧气;请参阅:阐明光合作用)。这一切使我们下意识地将生命和氧气联系在一起,当然其他的电子受体存在也是可能的
3. 如今不需氧的生命
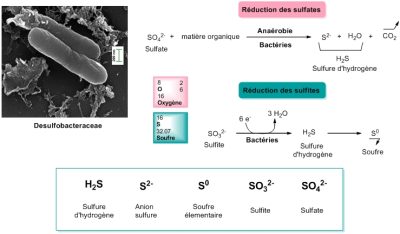
Desulfobacteraceae 脱硫杆菌科 Reduction des sulfates 硫酸盐还原,Sulfate 硫酸盐,matiere organique 有机物,Anaerobie 厌氧,Bacteries 细菌,Sulfure d’hydrogene 硫化氢, Oxygene 氧,
Soufre 硫,Reduction des sulfites 亚硫酸盐还原,Sulfite 亚硫酸盐,Bacteries 细菌,Anion sulfure 阴离子硫化物,Soufre elementaire 硫单质,Sulfite 亚硫酸盐,Sulfate 硫酸盐。
氧是一种很好的电子受体。上文提到的使用氧气的生物被归为“需氧型”(“需要空气”)。几乎所有多细胞生命以及单细胞生物都是需要氧气的。但也有其他类型的有机体是厌氧的(“无需空气”),它们使用其他最终电子受体。例如脱硫杆菌科的细菌使用硫酸根阴离子作为电子受体,将其还原为硫化物(图 3)。然而,它们也可以利用硫单质,在门捷列夫元素周期表中,硫元素位于氧元素的下面,它们是具有相似属性的元素(两个“硫族元素”),因此有机体使用硫元素也就不足为奇了。从某种意义上讲,既然缺氧,为何不使用氧的“表亲”硫元素呢?将硫还原成硫化物,生成硫化氢,这与氧气被还原为水的过程是十分相似的。
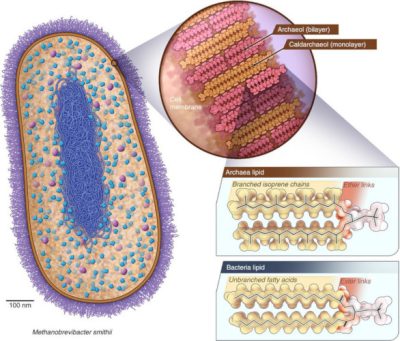
一些单细胞生物能够产生甲烷(CH4),它们被称之为产甲烷古菌,这些细菌不会还原氧或硫,但会还原二氧化碳[4]。地球大气中的大部分甲烷都是由这类古菌产生的。产甲烷古菌生活在河口、沼泽或荒地湿沉积物中(荒野上明亮的光可能是古细菌排放的甲烷燃烧产生的火焰)以及在极端环境中[5],如沸腾的温泉和黑色海底烟柱附近(请参阅:极端环境中的微生物)。另一些则生活在反刍动物和人类的肠道里,如史密斯甲烷短杆菌——一种产甲烷古菌——在人类肠道微生物中占比达10%[6](图4),这让人类也成为了甲烷的排放者。尽管人类一直在有氧环境下生活,但肠道内也存在无氧的生命过程。
更进一步,考虑到人类是多细胞有机体和多个单细胞有机体的共同组成或“共生体生物”(请参阅:生命对环境约束的适应&共生和寄生),新陈代谢主要是有氧过程,(很)少数是厌氧过程。
对于许多生活在无氧环境中的生物来说,氧气不仅没有用甚至是有害的。对于它们来说,氧气是一种能迅速摧毁它们的毒药,是死亡的代名词。
4. 最初无氧的生命
40 亿年前生命开始出现时,地球大气中并没有氧分子。早期大气的组成很难确定,人们最初认为它主要由氢气、甲烷和氨气组成,但这种组成的大气很容易被还原,特别是当氧气出现,与氢反应生成水(氧气被还原),大气会迅速消失。现在可以确定大气主要由二氧化碳和水蒸气组成,水蒸气是更容易将氧分子保留在大气中的一种介质。但陆地海洋的水中含有大量的亚铁离子(Fe2+),能够溶于水,任何痕量的氧气都会在将二价铁氧化成三氧化二铁(Fe3+),这是其最易被氧化的形式。
可以肯定的是,地球上的第一个生命是在无氧的环境中进行厌氧运作的(请参阅:生命出现的时代:40亿年前地球海洋化学)。如果地球上的生命出现在大约40亿年前(这种认知是合理的),在地球超过三分之一的历程中,氧不是任何生命的最终电子受体(氧化剂),那么什么是氧化剂呢?今天我们已经知道的硫酸盐、硫磺、氮衍生物、硝酸盐和亚硝酸盐,它们都可能是生命起源的潜在氧化剂。
但有一种至今仍然活跃的电子受体当时也是无处不在的,那就是二氧化碳。对于最初的氧化剂的角色而言,二氧化碳是一个重要的候选者。然后我们再回想下产甲烷古菌,如在我们肠道中运作的史密斯甲烷短杆菌,如果真是这样,那么40 亿年前地球上就已经产生了甲烷,但今天几乎不可能找到这种原始甲烷的痕迹,因为形成的分子都被回收利用,形成其他有机物和/或生成二氧化碳。即使存在少量的原始甲烷,它又怎么能和现在的生物甲烷区别开呢?也许是找别的地方?目前人们已经在火星上发现了甲烷。有人认为甲烷是在火星形成之初出现的,当时火星部分地表被海洋覆盖,生命开始在那里发展。在非常年轻的火星上,是否存在产甲烷古菌的类似物呢?我们离发现小绿人(小说里的一种外星人)还有很长的路要走,但是很有可能存在过火星人!生命消失后,火星上的甲烷可能一直保留到今天,将成为古菌的化石。
5. 氧气的历史及其对生物的影响
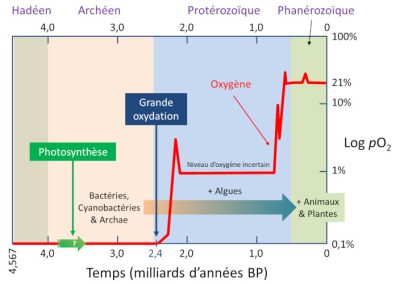
Hadeen 冥古代,Arcneen 太古代,Proterozoique 元古代,Phanerozoique 超生代,Photosynthese 光合作用,Grande oxydation 大氧化时代,Oxygene 氧气,Niveau doxygene incertaion 氧水平不确定,Bacteries Cyanobacterie 蓝藻细菌, Archae 古细菌,Algues 海藻类,Animaux 动物,
Plantes 植物,Temps(milliards dannees BP)年代表(十亿年)。
在生命出现的前三分之一时间中,仅存在原核单细胞生物(无细胞核)。由于现在存在细菌和古菌两个主要类别,我们有理由认为当时它们已经存在。在这些细菌中,蓝藻发挥着重要作用[7]。蓝藻在氧气产生这一地球史上最大的巨变中发挥了重要的作用。蓝藻至少在27亿年前已经存在了,最重要的是它们能进行光合作用,将二氧化碳作为原料制造出由碳原子组成的有机分子(糖),为了实现这一过程,它们利用太阳提供的光能。氧气是光合作用的副产物(请参阅:阐明光合作用),大量氧气的出现几乎逐渐氧化了所有可以还原它的物质(包括亚铁)。直到24亿年前,氧气含量升高并开始积累(请参阅:生物圈——主要的地质参与者)。
5.1. 大氧化事件:氧气增多
在24亿到20亿年前期间,地球出现了大氧化事件(GOE)[8]。这种“氧气危机”对大部分厌氧代谢生物来说是毁灭性的,这也许是地球历史上最大规模的物种灭绝,绝大数物种都消失了。微生物的大规模灭绝,虽然没有恐龙灭绝被人熟知,但其影响巨大。正如一些鸟类恐龙在白垩纪大灭绝中幸存下来,而其他恐龙都消失了一样,一些古老的细菌通过在非氧化生物群落中避难或通过适应,成功地度过了这些大氧化危机(因此原核生物仍然存在)。
在适应性过程中,生物进化得相当完善。在 GEO 之前,任何生物都不具有细胞核,到 GEO 结束时,一些生物进化出细胞核,真核生物就此诞生。有人认为真核生物是古菌是为了抵抗氧化应激而演化出的,它们会形成内膜,其中一层会变成核膜。在这层膜中,新物种的 DNA 会得到更好的保护从而免受氧气的攻击。然后,或者与此同时,细菌会来吞噬这些原始真核生物,也许在那里找到保护。这些具有细胞核和细菌宿主(如线粒体)的古生物是真核生物(请参阅:共生与进化:真核细胞的起源)。正是因为它们(以及有氧代谢的原核生物)的出现,才有可能将生命和氧气联系起来。
5.2. 富氧时代到来

在GOE之后,地球有了氧气,但含量比今天少得多。目前地球大气氧的含量约为21%,大概有10亿吨氧(请参阅:地球大气层和气体包裹层)。在GOE之后的10亿年里,氧含量占比一直较低且保持稳定,大约为3-4%。但在不到10亿年前,氧气含量又开始增长,可能达到30%以上。当时地球上的生命,包括动物、植物、蘑菇,以及大量的真核生物和多细胞物种开始涌现。
氧气有效含量的显著增加和大型多细胞物种的繁殖,这两个事件很可能是存在联系的。这种增加是光合作用以及沉积物中产生氧气的有机物的捕获和其他耗氧现象之间的相对变化的结果,例如岩浆中铁离子的氧化、矿物硫、古老的沉积岩等的氧化。
消耗氧气的有氧呼吸提供了大量的能量,使生命爆发成为了可能。至少在地球,氧气就是生命,即使氧气过量。也许是由于3亿年前二叠纪氧气含量较高(现在空气中氧气含量只有21%,曾经有23%),出现了一种30厘米长,翼展70厘米的巨型蜻蜓(图 6))。提出该假设的作者认为今天的地球上没有足够的氧气来支撑巨型蜻蜓生存。现在最大的蜻蜓的翼展只有20厘米(这已经很不错了)。
5.3. 氧气含量过多?
从冰河时期开始,过多的氧气会导致严重的问题。甲烷是一种强大的温室气体,如果含量不是很高的情况下就会留在大气中。在地球上甲烷可以维持一种叫做“全球热带”的气候,氧气含量的增加将导致它被氧化成二氧化碳。二氧化碳(现在人们总在谈论二氧化碳,这是对的,因为现在人们面临着全球变暖的形势)也是一种温室气体,但温室效应强度比甲烷小得多(每单位质量比甲烷低20多倍)。因此,如果甲烷被大量氧化成二氧化碳意味着要地球气候将变冷,因此7亿到6亿年前的大型冰川开始出,这个时期被称为成冰纪(来自于古希腊语:寒冷)。
可以想像,在这个期间整个地球表面都被冻结了,形成了一个“雪球”。这个时期之后即埃迪卡拉纪,多细胞生命才真正占据了主导地位(请参阅:第一个复杂的生态系统)。埃迪卡拉纪的动物区系极其丰富多样,但在5.45亿年前完全消失了,取而代之的是一种新的动物群——寒武纪生物群,是我们今天已知生物的来源。海洋表层海水中氧气含量增加,可能是导致埃迪卡拉纪动物群的爆发的主要原因,由于海水层的不稳定,促使有毒气体硫化氢上涌,最终导致了当时生物群的突然消失。
Oxygen氧气,Heat加热,Fuel燃料。
氧气还有另一个主要缺陷:它能燃烧有机物,每年的火灾都会摧毁相当大面积的森林和荒地,几乎会摧毁所有的植物和动物。空气中21%的含量的氧气足以点燃木材,尤其是在干燥或不太潮湿的情况下。如果氧气含量更高,那么火灾破坏性更强,对整个生态系统来说是十分危险的(图7)。
六千五百万年前,当一颗巨大的陨石撞击墨西哥尤卡坦半岛,导致所有非禽类恐龙的消失(请参阅:地史时期的大规模物种灭绝事件),那时空气中氧气的含量比今天高出了几个百分点。但百分之几的含量差能产生了十分重要的影响。尤卡坦半岛的恐龙(和其他动物)最早消失,撞击冲击波传到了北美和南美,波峰的前部呈圆型散开并压碎了沿途的所有物体。引发的巨大的海啸淹没了所有海拔低的地方,甚至波及到了非洲和欧洲。
到目前为止,空气中高浓度的氧气与海啸等并没有关系,但后来,发光的火焰灰烬如雨点般撒落在星球上。地球上发生数百万次的大火,这些火很快汇聚在了一起,然后遍布了整个地球。当时比现在高出的百分之几的氧气给恐龙带来了巨大打击。许多恐龙都生活在湿地中,如果按照如今的氧气含量,其中的森林有可能不会燃烧,至少一些红树林和沼泽地不会被烧毁。与恐龙最初的数量相比,能存活下的恐龙的数量可能很小但也不容忽视,它们在这一阶段存活了下来。太阳光无法穿越火灾引起的烟雾,无法到达地面,导致随后气温降低,也许存在一些恐龙能够适应随后的寒冷气候。可以肯定的是,过多的氧气是危险的。
6. 不稳定的平衡
正如我们所看到的,不加区别地将生命和氧气联系起来是错误的。不需要氧气的生物过去一直存在,且现在仍然存在(甚至在人类体内)。在过去地球三分之一的历史中,生命是不需要氧气的。当蓝细菌(一种制氧细菌)大量繁殖时,海洋和大气层中氧气增加,当时这可能会成为一场严重的地球危机。幸运的是大氧化事件持续时间很长(几亿年),细菌和古菌有充足的时间适应这种环境,进化使具有细胞核并与细菌相关的古菌得以选择(数量上的优势!)。真核生物就此诞生,但它们要花很长时间才能演化成动植物,同时氧气会促进这一过程,但氧气能够燃烧生物的麻烦依然存在。
陆地上的氧在一个巨大的地球化学循环中被吸收,通过生物的呼吸、分解、埋藏、碳酸盐(石灰岩层)或碳氢化合物和煤藏的形成,与其他元素(主要是碳)的循环相联系。它还通过形成硫酸盐与硫循环有关。因此氧水平的稳定既是许多化学平衡的结果,也是物理过程的结果。如果海洋中产生的所有有机物质都被氧化,海洋就不会释放出任何物质。实际上,正是因为有机质的一小部分在被氧化之前就被埋藏了,所以平衡才保持为正。这种平衡是非常不稳定的,保持平衡的大概在0.0001%以内[9]!
回到砍伐森林的问题上来,对于亚马逊雨林的生物多样性保护,尤其是对于人类的生存来说,砍伐面积与日俱增不是一个好主意。但亚马逊和世界上其他所有的森林并不是地球的“肺”[1]。树木可以产生氧气同时也在消耗氧气,因此整个过程是平衡的(请参阅:生物圈——主要的地质参与者),只有幼树吸收二氧化碳比释放的多。地球大气中的大部分氧气来自海洋,确切的说来自构成浮游植物的微生物。我们在浮游植物中(紧邻真核藻类)发现了蓝藻,浮游植物中的所有原核生物都是蓝藻,而蓝藻是造成大氧化事件细菌的后代。如今由于海洋变暖,蓝藻生存环境正处于危险之中。海洋的氧平衡之所以是正的,是因为海洋中一小部分有机物沉淀到海底,因而没有被氧化。因此与其说是海洋产生了氧气,不如说是氧气部分“非消耗”的结果。但如果想保留下更多氧气,首先必须保护蓝藻(图8)。
7. 需记住的信息
- 从本质上说,是地球化学和地质现象决定了大气中氧的含量。
- 10多亿年来,地球上的生命是在缺氧的环境下发展起来的。
- 大约20多亿年前,氧气的出现,也就是大氧化,是一个重大事件彻底颠覆了地球上的生命。这时,真核细胞(有细胞核)出现了。
- 当氧气含量再次增加到20%到30%时,我们今天所知道的多细胞生命出现并多样化(动物、真菌、植物)。
- 氧是当今生命所必需的,但它是一种强大的氧化剂。过多的氧气可能不利于生命的维持,或许亦会对生命带来巨大变化。
参考资料及说明
封面照片:大船底座星云中的氧气图像[图片来源:迪伦·奥唐纳的原始照片;托比亚斯·弗雷/CC0的衍生作]
[1] Zimmer, Why the Amazon doesn’t really produce 20% of the world’s oxygen ? National Geographic, 28 Août 2019
Zimmer K.,为什么亚马逊河流域不能生真正产生世界上20%的氧气?国家地理,2019年8月28日
[2] Oxygène (Futura Sciences) 氧气(未来科学)
[3] Réaction d’oxydo-réduction: un transfert d’électron (La chimie.net) 氧化还原反应:电子转移(La chimie.net)
[4] Les archées: rencontre du troisième type (MNHN). 拱门:第三类会议(MNHN)
[5] Boisson, Feux follets: que sont en réalité ces étranges lumières? Trust my Science,2018,Boisson T., Feux follets 这些奇怪的光到底是什么?相信我的科学,2018年
[6] Gootlieb K., Wacher, Sliman J.&Pimentel M., AP&T,2016,43,197, DOI:10.1111/apt.13469 Gootlieb K., Wacher V., Sliman J.&Pimentel M., AP&T,2016,43,197, DOI :10.1111/apt.13469
[7] Claire König. Les cyanobactéries: apparition, adaptation et reproduction. (FuturaSciences) Claire König 蓝藻的出现、适应和繁殖(未来科学)
[8] The event that transformed Earth(BBC). 改变地球的事件(BBC)
[9] Field C.B., Behrenfeld M.J., Randerson T.&Falkowski P., Primary Production of the Biosphere:Integrating Terrestrial and Oceanic Components. Science,1998,281:237-240. DOI:10.1126/science.281.5374.237 Field C.B., Behrenfeld M.J., Randerson J.T. & Falkowski P.,生物圈初级生产:陆地和海洋组成部分的综合。 科学,1998,281:237-240. DOI:10.1126/science.281.5374.237
环境百科全书由环境和能源百科全书协会出版 (www.a3e.fr),该协会与格勒诺布尔阿尔卑斯大学和格勒诺布尔INP有合同关系,并由法国科学院赞助。
引用这篇文章: LEQRAA Naoual, VALLÉE Yannick (2024年3月13日), 氧气与生命之间的危险联系, 环境百科全书,咨询于 2025年1月22日 [在线ISSN 2555-0950]网址: https://www.encyclopedie-environnement.org/zh/vivant-zh/the-dangerous-liaisons-of-oxygen-and-life/.
环境百科全书中的文章是根据知识共享BY-NC-SA许可条款提供的,该许可授权复制的条件是:引用来源,不作商业使用,共享相同的初始条件,并且在每次重复使用或分发时复制知识共享BY-NC-SA许可声明。






