空气污染
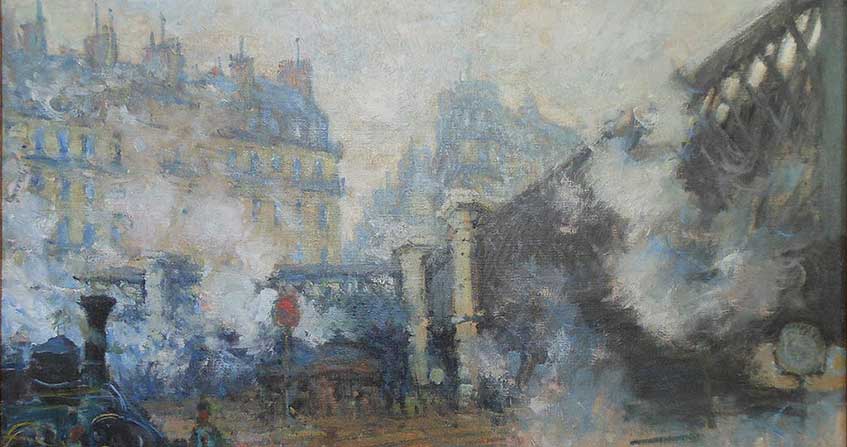
空气污染类型多样且历史久远,不仅会影响人类健康,还会影响生物圈,特别是会影响地球的植被、大气和气候。空气污染源包括自然产生的污染物,以及具有相同性质的人类活动释放到大气中的污染物。本文概述了空气污染的历史,其中包括一些著名的污染事件。在局地尺度污染的影响主要表现为健康影响,在区域尺度表现为森林锐减和跨界污染,在全球尺度表现为平流层臭氧层的破坏、温室效应和全球变暖。就对健康的影响而言,室内空气污染、自然或人为造成的放射性污染(如最后一次大气层核试验与和平利用核能有关的事故)都不应被忽视。在众多污染类型中,有些空气污染已经得到了基本的控制,如酸雨和平流层污染。
1. 一次污染物和二次污染物
1996年12月30日的《法国空气和合理使用能源法》对空气污染做出了定义(详情参考法律如何保护空气质量?)。直接由人类活动、工业、生活、农业、交通运输和燃烧等活动引起的。通过以上方式释放的污染物称为人为污染物。此外,生物圈也会自发地向大气释放气体和颗粒物,如风蚀过程会生成颗粒物、植物会释放碳氢化合物、土壤和水体中细菌的分解会产生气体,携带清洁剂的海水会释放浪沫,这些要素的成分(见图1)与污染物具有相同的性质,被称为生物污染物。人类活动改变了污染物的释放形式,导致了空气污染。如农业上人们利用氮肥,改变风蚀和氮氧化物等气体的释放过程,破坏了大自然的氮循环。
直接从污染源排放至大气的,或由生物圈自然释放过程变化所引起的污染物,被称为一次污染物。许多一次污染物会发生化学反应——特别是在太阳辐射的影响下,生成新的成分或二次污染物。二次污染物通常比一次污染物更具有环境危害性。二次污染物包括强酸,如硫酸和硝酸,以及臭氧(O3)等强氧化剂。因此,大气是强烈化学活动发生的场所,参与反应的化合物大多是微量的。大气中活性成分的浓度[1]通常用体积混合比来表示,体积混合比可以很小,如 ppt级,即每1012(或1万亿)空气体积中有一个污染物体积。通常是ppb(十亿分之一,b指英语单词billion)或ppm(百万分之一)级。
2. 空气污染简史
空气污染是一个非常古老的现象,在古代即有所显现。早在公元前61年,塞内加就曾抱怨罗马的空气污染。造成污染的部分原因是城市和地区人口的聚集,伴随着用于家庭、工业或其他用途的各种材料大量燃烧。在伦敦,煤炭燃烧早在12世纪就造成了空气污染问题。1273年,议会禁止在该地区燃烧煤炭。14世纪初,爱德华一世想要限制燃煤的使用。16世纪,伊丽莎白一世女王禁止在议会会议上使用煤炭。1661年,英国作家兼回忆录作者约翰·伊夫林(John Evelyn)发表了一篇关于空气污染的论文《驱逐烟气》(Fumifugium),该论文于1960年由国家清洁空气协会重新发布。他主张把污染工业从伦敦市中心移到周边地区。19世纪,小说家查尔斯·狄更斯(Charles Dickens)对伦敦和其他英国城市的烟雾进行了详尽的描述。卡米尔·弗拉马里昂(Camille Flammarion)在1872年出版的有关大气的书[2]中,强调巴黎糟糕的空气质量及其对健康的有害影响(请参阅献给这位作者的焦点中的引文)。

从19世纪末到20世纪中叶,污染随着工业生产的增长而增加,在缺乏约束性法规的情况下,排放到大气中的气体也随之增加。许多著名的画家都用排放污染物的烟囱来代表工业或城市场所,如封面图片和图2所示。20世纪中叶,空气污染事件显著提高了死亡率和发病率,人们开始意识到空气污染对人类健康的危害。
3. 若干严重污染事件
1930年12月1日至5日,由于反气旋现象,在比利时东部列日市(Liège)附近的马斯河谷出现了一次强烈的逆温,伴随大雾持续了5天之久。排放的众多污染物,特别是二氧化硫(SO2)和颗粒物,在宽1公里,深100米的山谷中积聚。当时没有污染物检测,因此浓度水平不明。最终,大雾导致一些二氧化硫转化为硫酸。据报道,该污染事件造成了多人死亡(共计63人,正常情况下为6人),数百人中毒。
1948年,位于美国宾夕法尼亚州,离匹兹堡约50公里的山谷中的工业小镇多诺拉(Donora)发生了一起严重的污染事件。10月27日至31日,来自各种工业设施的污染物累积。该事件导致20人死亡(正常情况下为2人)和数千人中毒。如同马斯河谷事件,逆温层的存在伴随着雾的形成(详情参考《山风》),迫使受污染的冷空气滞留在山谷底部,促使二氧化硫转化为硫酸。
其中最严重和最引人注目的事件发生在20世纪上半叶的伦敦。为了表示烟和雾的混合物,引入了烟雾[3]这个词。洛杉矶盆地的高污染,加上沿海地区常伴海风,极大地提高了人们对空气质量下降及空气污染对健康影响的认识(见图3)。
除了上述慢性污染之外,还有意外污染,大量释放有毒物质,造成多人受害(博帕尔毒气泄漏事件、塞韦索污染事件、切尔诺贝利事件等)。
4. 局部地区的主要污染物
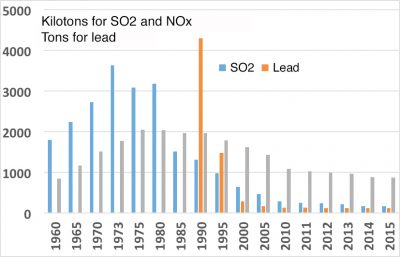
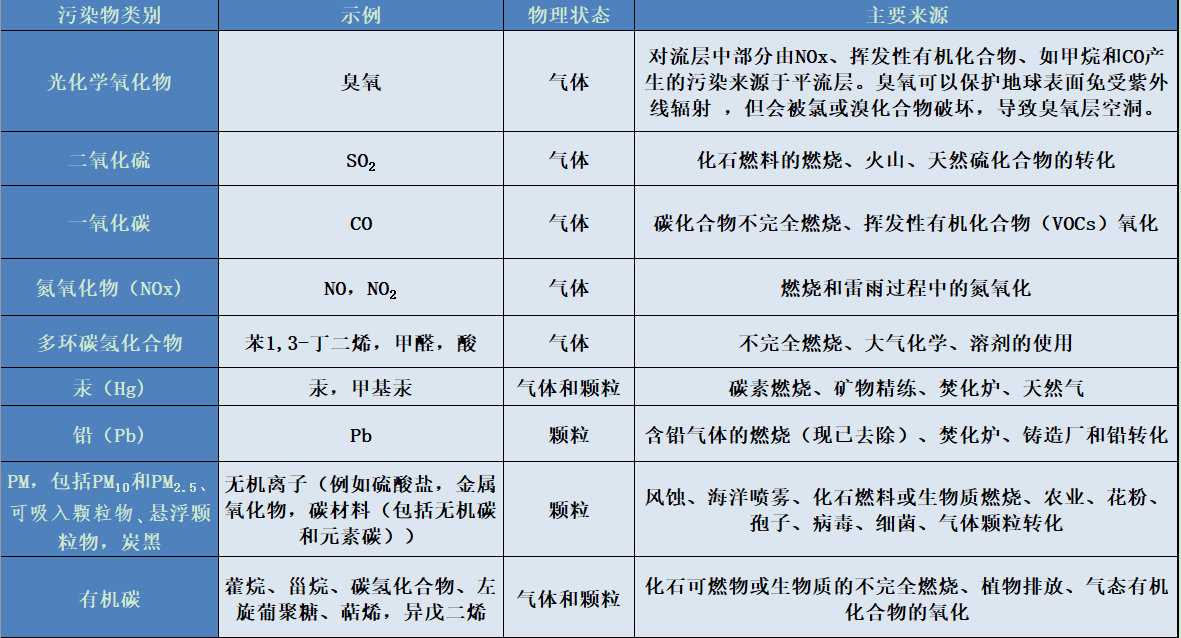
当前,导致局部地区空气污染主要的一次污染物是固体颗粒(详情参考空气污染颗粒究竟是什么?)氮氧化物(NOx)和碳氢化合物。附表给出了它们的性质、来源:汽车、轻型车辆和重型货车发动机中燃料的燃烧或蒸发,燃烧木材或燃料取暖等。
目前一些主要污染物的排放量和浓度已经显著下降(见图4)。如二氧化硫(SO2)就是这种情况,它来自燃料(煤、燃料油等)中所含的硫、铅,用于增加汽油的辛烷值,及其他重金属,如主要由废物焚烧炉释放的汞和二恶英等。此外,生物源化合物,如花粉、孢子、细菌等,必须列入一次污染物列表中。
主要污染物类别及其物理状态和来源表
5. 区域性污染:酸和氧化剂
上述污染事件发生在局地区域或较小的范围,规模和影响范围有限。大气污染也发生在区域层面(从十几公里到几百公里甚至一千公里),被称为中尺度气象。浓度水平不如局地高,且由于是二次污染物导致,产生的影响会更隐蔽。在1970年代及随后的几年中,在欧洲和美国的某些地区发现了森林梢枯病。空气污染,特别是降水和地下水的酸化,以及空气氧化能力的提高被认为是导致该病发生的原因。这种污染不分国界,通常被称为跨界污染。每年夏季监测到的高浓度臭氧可归类为此类区域跨界污染。
6. 二次污染物
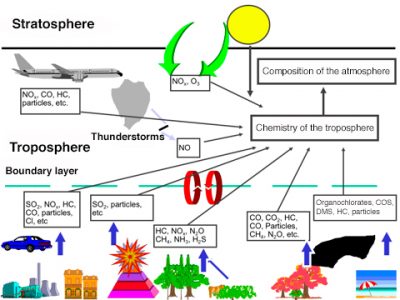
一次污染物向二次污染物的转化可以发生在气相中,其中太阳辐射起非常重要的作用,这种转化也可以发生在雾和云中的液相中。大气化学中主要的二次化合物是羟基自由基OH,有时被称为大气清洁剂。直到1970年代初,羟基自由基才引起人们的重视。羟基自由基主要是臭氧光解形成的。由于其反应活性较高,大气中羟基自由基的浓度非常低。它能将二氧化硫转化为硫酸,也可以调节一氧化碳(CO)、甲烷)CH4)和其他碳氢化合物等化合物的浓度。污染物在大气中的停留时间取决于它们浓度,而浓度又取决于反应。在碳氢化合物存在的情况下,羟基自由基会产生比自身更具有活性的化合物。图5总结了低层大气中从污染源到受污染地区范围内的所有污染。
7. 全球污染:温室效应,臭氧层空洞
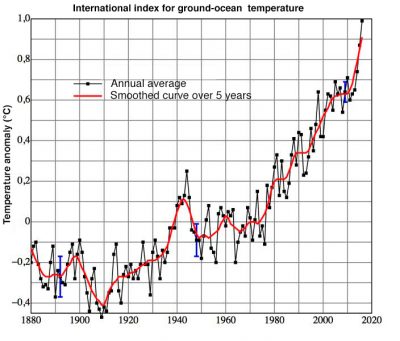
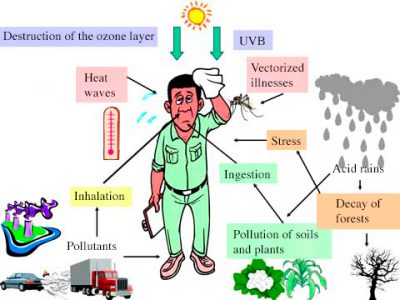
大气污染会影响整个地球,主要表现在两个方面。其一是平流层臭氧(O3)层的破坏[4](详情参考地球的大气层和气体层),会增加到达地表的紫外辐射光对人类和生物圈的危害(详情参考太阳光中的紫外线对细胞的影响)。其二是被排放到大气中的有些低活性气体会在对流层中不断积累导致浓度升高。太阳辐射可以穿透这些气体,一小部分被地表吸收。吸收的部分能量以红外辐射的形式释放出来,部分被这些气体吸收,能量被大气捕获从而导致低层大气变暖,这种现象被称为温室效应。大气起着跟温室的玻璃或塑料墙一样的作用。大气中水蒸气和二氧化碳等吸热气体的存在,使得温室效应在大气中自然而然地发生。大气中甲烷等其他成分,对温室效应也起到了显著的影响,不过影响力不及水蒸气和二氧化碳。正是因为温室效应地球才有了温和的温度。如果没有温室效应,地球表面的平均温度将比我们目前的温度低35摄氏度左右(详情参考气候机器)。自工业时代以来,污染已经显著增加了温室效应的强度,虽轻微但足以引起地球气候[5]变化,如图6所示。这被称为额外的温室效应。
综上所述,大气污染有三个不同的尺度。在局地尺度,它主要影响人类健康,却又不仅局限于此;在区域尺度,它造成的显著危害是地表水的酸化和森林的锐减;在全球尺度,随着平流层臭氧层的破坏和温室效应的加剧,大气污染会导致气候变化,对生物圈、海平面和人类健康都有影响。
8. 室内空气污染
除这三种主要污染类型外,还有室内空气污染[6]。在工业化国家,人们平均80%的时间待在工作场所和生活场所。污染的空气会进入室内,室内还有很多其他污染源[7],有些室内污染物具有跟室外污染物相同的性质。室内空气污染可能比室外大气污染更严重。然而,不同环境差异很大,因此研究室内空气污染及其影响较为复杂。
9. 空气放射性和人为污染
此外,室外大气和室内空气均含有放射性污染物、气体和固体颗粒。这种放射性污染物是地质来源(详情参考放射性与核反应),它来自于属于铀和钍放射性家族的稀有气体排放。在某些地区,放射性污染的浓度可能很高,超过铀矿允许的浓度值。
在二十世纪五六十年代,美国、俄罗斯、英国、法国和中国在大气层中进行的实验性核爆炸,向大气排放了大量主要来自铀235或钚裂变的物质。核能和平利用时发生的反应堆事故也能向大气释放放射性物质。1986年4月26日发生在乌克兰的切尔诺贝利事故是最悲惨、最典型的。包括法国在内的欧洲大部分地区的大气层中弥漫着各种放射性物质,其中包括铯137和碘131(详情参考农药:历史给我们的教训)。此外,2011年日本发生了地震,继而引发海啸。该地震导致福岛核事故,导致几个反应堆的冷却系统失灵,放射性物质释放到大气及周边环境中,特别是海洋中。
10. 空气质量演变
目前全球范围内环境质量已经有所改善,但仍有很大的提升空间。由于水蒸气的凝结,工业和城市的烟羽仍然可见。这并不意味着没有更多的污染物,然而污染物处理系统可以去除其中的大部分。国际协定大大减少了对平流层臭氧层的破坏和降水的酸化。希望它同样能减少温室气体的排放。然而,大气中氮氧化物、颗粒物浓度很高,与底层大气中臭氧浓度一个水平。第一个主要的国际行动是1992年的里约热内卢会议。2016年巴黎会议作出很多鼓舞人心的承诺,但尚未落实。
参考文献及说明
封面图片:克劳德·莫奈的一幅画,描绘了20世纪初巴黎的空气污染。(圣拉扎尔桥,玛摩丹美术馆)
[1] 无论是气体污染物还是颗粒污染物,其浓度都是以每单位体积的质量来表示的,通常以微克每立方米来表示(μg/m3)。对于气体,则并不使用浓度表示,而是也经常使用混合比,通常是体积比,代表空气中污染物的体积比例。用ppmv(百万分之一)或者ppbv(十亿分之一,ppt(十亿分之一或1012分之一))表示。很多时候不用v(体积)表示,用ppm,ppb或ppt。1 ppm的混合比意味着每百万个空气分子中有一个污染物分子。
[2] Camille Flammarion, L’atmosphère, Librairie Hachette, 1872.
[3] 这个英语新词“smog”就是语言学家所说的“手提箱词”。它由“smoke”和“fog”这两个词的合并而成。
[4] 平流层臭氧是由短波紫外线太阳辐射引起的氧气光解产生的。这种放热化学反应能解释对流层上方 20 至 70 公里高度之间的空气变暖现象。
[5] 全球平均温度包括大陆和海洋表面温度的大量观测值。2016年全球大陆和海洋表面平均值温度比20世纪高0.94°C,2015年高0.90°C,2014年高0.74°C(www.ncdc.noaa.gouv/sotc/global/201613)。
[6] Luc Mosquerons and Vincent Nedellec. Indoor Air Quality Observatory. Inventory of French data on indoor air quality in buildings. http://www.oqai.fr/userdata/documents/Document_16.pdf
[7] YEARS – Indoor Air Quality. https://www.anses.fr/fr/content/qualité-de-l’Indoor Air
环境百科全书由环境和能源百科全书协会出版 (www.a3e.fr),该协会与格勒诺布尔阿尔卑斯大学和格勒诺布尔INP有合同关系,并由法国科学院赞助。
引用这篇文章: FONTAN Jacques (2024年3月5日), 空气污染, 环境百科全书,咨询于 2025年1月29日 [在线ISSN 2555-0950]网址: https://www.encyclopedie-environnement.org/zh/air-zh/air-pollution/.
环境百科全书中的文章是根据知识共享BY-NC-SA许可条款提供的,该许可授权复制的条件是:引用来源,不作商业使用,共享相同的初始条件,并且在每次重复使用或分发时复制知识共享BY-NC-SA许可声明。