空气污染颗粒究竟是什么?

颗粒物是空气污染物的主要成分,是导致空气污染死亡的主要原因。颗粒物细分不同种类,具有不同的物理、力学属性,人们对此知之甚少。本文旨在让人们更好地了解大气中颗粒物的性质及其引发的问题,尤其是对健康的影响。
1. 我们在谈论什么?
1.1. 颗粒和气溶胶

[来源:SeaWiFS项目、美国宇航局戈达德太空飞行中心(NASA/Goddard Space Flight Center)及ORBIMAGE(美国航天局可视地球),来自维基共享资源]
颗粒往往被误称为气溶胶。事实上,气溶胶是气体(通常是空气)和细小的固体或液悬浮颗粒形成的混合物(见图1)。因此,颗粒物是气溶胶的成分之一。颗粒物的沉降速率必须很低才能悬浮于气体中。通常认为气溶胶的球形颗粒的直径小于 100 µm或0.1mm。
1.2. 用于区分颗粒的术语
人们使用不同的术语来描述特定类型的气溶胶,误用情况常有发生。
- 灰尘是一种固体颗粒,通常直径大于1µm。在风蚀等机械过程中悬浮(例如:沙尘、灰烬、路尘、花粉等)。
- 烟通常是指由燃烧产生的细小颗粒。英语中的fume一词也指蒸汽凝结产生的细小颗粒。
- 雾(雾和烟雾)是由蒸汽凝结或喷雾形成的悬浮液滴。在近地面大气中,当能见度小于1公里,颗粒尺度通常大于5µm。人们会说“起雾”了。
- 薄雾(薄雾或霾)由较小的液滴或固体颗粒组成。与雾相比,霾的能见度更低,但沙漠或半沙漠地区的霾除外。
需要指出的是,在该领域中英语的表达比法语更丰富。法语单词“brume”可以翻译为英文的haze(霾)或mist(薄雾)。“smog”一词由“smoke”和“fog”组合而成,译为法语是brouillard photochimique”。
1.3. 缩略词PM
PM是颗粒物质(particulate Matter)的缩写。PM10、PM2.5指存在于大气中粒径分别小于10µm和2.5µm的颗粒。采用这些定义是为了考虑颗粒物在吸入过程中的表现。一般情况下,直径大于10µm的颗粒大多滞留在鼻腔中,这种颗粒是不可吸入的。直径小于2.5µm的被称为细颗粒,直径介于2.5µm 和10µm之间的颗粒被称为粗颗粒。4.2节对颗粒直径有更详细的描述,上述的颗粒直径其实指的是“中位粒径”,大约50%的颗粒小于中位粒径。
1.4. 其他术语
云凝结核(CCN):又称凝结核,是使水蒸气凝结为液态时作为凝结核心的颗粒,其直径至少大于0.1µm。
黑烟:黑烟是一种大气颗粒,一般使用过滤器收集。可以通过光学方法确定过滤器的反射率进而测定黑烟含量。这种测定方法长期用于检测空气污染。
烟灰是碳颗粒,通常来自燃烧过程中碳氢化合物的凝结。柴油发动机所产生的颗粒中含有大量烟灰。
Aitken核:指的是直径小于0.1µm的颗粒,目前已经很少使用这一名词。Aitken核又被称为纳米粒子或超细粒子(参见文章:受到控诉的木材燃烧和柴油发动机)。
2. 大气中的颗粒
2.1. 一次污染物和二次污染物
颗粒包括一次污染物和二次污染物(参见文章:空气污染)。它们可以自然排放到大气中,也可通过气态污染物之间的化学或光化学反应产生,或由大气中的水汽凝结形成。一次污染源多种多样,既有人类活动造成的,也有火山喷发、风蚀、海浪飞沫、花粉、植物碎屑等自然颗粒造成的。人类活动可以影响这些自然排放。
2.2. 颗粒对大气的影响
颗粒通过散射和吸收来改变大气的光学特性。空气污染的表现之一就是能见度降低。颗粒不仅可以进行逆向散射,也可以吸收来自地面和大气的太阳能、红外辐射能量,进而影响大气的辐射平衡。颗粒也参与了云和雾的形成。如果没有颗粒,生成水滴和冰晶的凝结和结冰过程则需要更低的温度。均质冷凝指的是没有凝结核的冷凝过程,这一过程需要500%的过饱和度;均质升华需要3000%的过饱和度;均质冻结需要-40℃的温度。然而,在颗粒存在的情况下,湿度低于100%时就会发生冷凝。因此,云中液滴的数量及其大小受到充当凝结核的颗粒的影响。大陆云的凝结核比海洋云的凝结核数量更多、直径更小。大陆云包含了较多、较小的水滴,而海洋云则包含了较少、较大的水滴,因此大陆云不易形成降水。
2.3. 如何表征颗粒特性
要了解空气污染颗粒的影响,我们必须根据最终的目标以不同精度表征颗粒特性:
- 颗粒浓度:可按情况用单位体积的质量、表面积或颗粒数来表征。
- 颗粒化学性质:一般在空气污染监测网络中,我们只对颗粒质量浓度进行系统测量,而流行病学研究则经常使用颗粒浓度这一概念。
- 颗粒的理化性质主要取决于它们的粒径。第3节根据颗粒直径大小介绍了粒径谱。测量绘制粒径谱需要昂贵且复杂的设备。
2.4. 定义颗粒大小
我们通常用直径或者半径来描述球形颗粒的大小。
无论颗粒呈现何种形状,当我们使用光学或电子显微镜观测颗粒时,有若干种定义颗粒大小的方法。通常使用能够包含该颗粒的球的直径,或至少能包含颗粒在观察面上投影的圆的直径来表征。
往往有多种原因不能使用显微镜来观察颗粒:首先,在介质上收集颗粒时可能会存在粒径变化。颗粒表面存在的凝结水汽会改变颗粒与其周围介质之间的平衡。电子显微镜要求将颗粒置于真空中,然后进行电子轰击,这会导致凝结水汽的蒸发,从而改变颗粒的特性。再者,光学显微镜的使用仅限于直径大于0.1µm的物体。
为了确定颗粒大小,我们需要测量颗粒的物理特性,这些物理特性取决于颗粒的大小。例如,热扰动系数(或布朗运动)、光散射、电场中的迁移率、沉降率等。假定颗粒是球形的,其粒径等于具有相同物理特性颗粒的直径。
颗粒通常以其平均直径来表征,但不同的作者对平均粒径有不同的定义。可以是算术平均值或几何平均值,其定义在统计学中是标准的。
3. 粒径谱
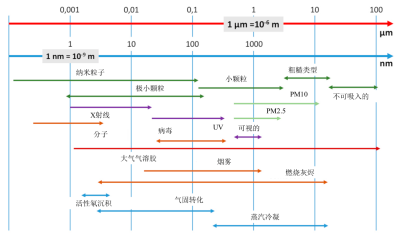
粒径谱由分布函数表示,该分布函数将质量(dM)、表面积(dS)、颗粒数(dN)在小尺寸范围dD中的比例表示为尺寸D的函数。分布函数通常需要归一化,即dM、dS、dN分别除以总质量M或总面积S或粒子总数N。
[来源:© J. Fontan]
如图2所示,粒径主要分布在µm、nm等几个数量级上。线性刻度并不能充分表示细颗粒,细颗粒大多集中在代表直径的轴的原点。为了避免这种表征方法所存在的问题,我们倾向于使用直径(dD)的对数[1]来表示。
质量、表面积或颗粒数的分布函数由不同的曲线表示。以气溶胶为例,其粒径分布在不同数量级上,例如0.01µm或100µm之间,因此这三种分布中的任何一种都不能单独代表整个粒径分布函数。
[来源:©J.Fontan]
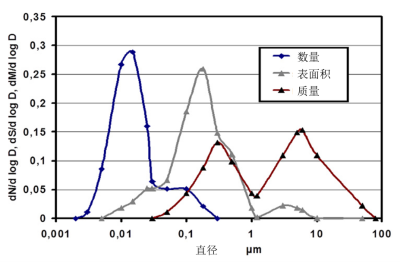
由图3可知,质量浓度的测量无法考虑到最细小的颗粒。这些颗粒数量最多,但与数量较少的较大颗粒相比,其质量是微不足道的。反之亦然。数量浓度的测量无法考虑到较大颗粒,因为这些颗粒数量很少。
根据前文所述关于粒径谱的局限性,我们不能仅仅测量颗粒的数量,也需要测量颗粒的质量和表面积。气体中颗粒发生的化学反应往往取决于颗粒表面的理化性质,因此表面积测定是非常重要的。我们需要选择颗粒大小分布以及合适的粒径测量方法。
如果说最细小的颗粒对人体健康危害更大,那么仅测量质量浓度就存在一定的局限性,应该同时测量颗粒的数量和质量。目前,用于规范发动机燃烧排放的欧6标准考虑了纳米颗粒的数量(参见文章:柴油发动机和木材燃烧;空气污染:了解信息,预防污染;法律如何保护空气质量)。然而,空气质量监测网络并未对这些排放物进行测量。
4. 颗粒的主要特性
颗粒的性质比气体分子的性质要复杂得多。为了了解颗粒的特性及其危害,我们必须了解它们的一些主要力学和物理属性:重力、惯性、热扰动、布朗扩散作用下的沉降速率等。这些特性对于颗粒在管道(呼吸系统)中沉积,过滤和颗粒凝并起着非常重要的作用。
4.1. 沉降速率
悬浮在气体(非湍流)中的颗粒在重力作用下降落的速率称为沉降速率。颗粒在自身重量和阿基米德推力的作用下很快达到一个垂直速度,即沉降速度。这个速度取决于半径的平方和比重(密度ρ)。一般情况下,我们是不知道颗粒的密度或形状的。这时,我们可以假设它是密度为1,空气动力学半径为ra的球形颗粒(ra代表等效粒径),其沉降速率与密度未知的实际颗粒相同。当颗粒的半径大于1μm时沉降速度才会变得明显。对于半径为50µm(直径100µm)的颗粒,其沉降速率可达到1000m/h。这一概念十分重要,因为超过这个速率,颗粒就不能再被认为是悬浮在气体中,气溶胶自然也无从谈起了。
4.2. 惯性
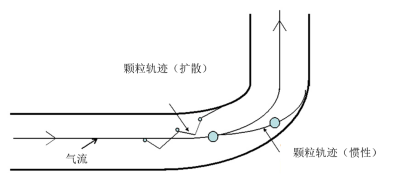
[来源:©J.Fontan]
当管道变窄,变宽或拐弯时,空气中的气流会发生形变,质量大于气体分子的颗粒不会继续跟随气流运动,它们会沿着最初的方向继续运动。然而,由于惯性,这些颗粒会撞击管壁,并聚集在管壁上或气流中的障碍物上(见图4)。这种现象的出现取决于三个要素:1)气流流速很高;2)颗粒质量大于空气分子的质量;3)气流突然偏离原始方向。这种现象主要发生于直径大于1µm的颗粒上。这种特性,即惯性,常用于在级联撞击器或旋风分离器中根据颗粒大小不同对其进行分离。颗粒分离并不是基于特定颗粒的直径,而是基于“中位粒径”,其中约50%的颗粒小于中位粒径。惯性可以在采样过程中区分PM10和PM2.5并去除粒径最大的颗粒。因此,当我们基于中位粒径讨论PM10或PM2.5时,并不意味着所有颗粒的直径都小于10µm或2.5µm。
4.3. 布朗扩散
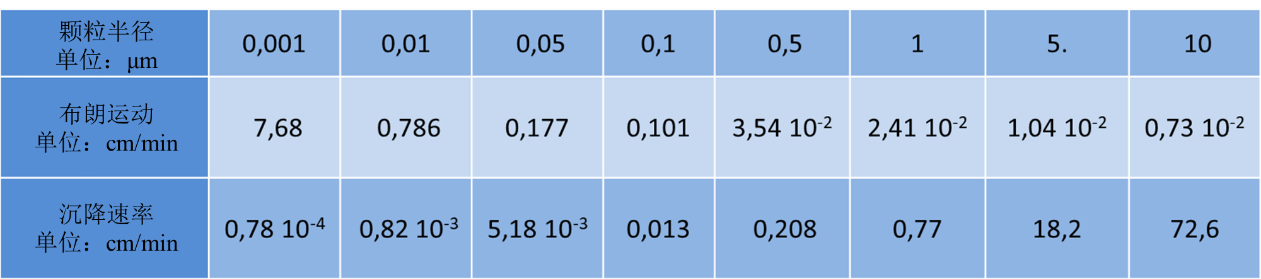
表1. 布朗运动与沉降的对比
布朗扩散是粒子与气体分子碰撞后产生的不规则运动。表1显示了一秒钟内投射到轴上的平均位移。当粒子的空气动力学半径小于0.1µm时,这种位移就会变得明显。而且比重力引起的位移更重要。这是细小颗粒的一个重要特性。在封闭区域、管道和呼吸道中,由于这种布朗运动,颗粒会沉降在壁上。在过滤器中,在布朗运动作用下,非常细小的颗粒会被有效拦截,更容易被收集起来。这看起来似乎有些矛盾。常有人说极小的颗粒很难被拦截,但这种说法是错误的。
根据Fuch[3]的研究,在常温常压条件下,表1显示了密度为1的球形颗粒进行布朗运动和自由沉降的速度对比(单位:厘米/分钟):表1第二行是布朗运动的结果,表1第三行是自由沉降速率。当颗粒非常小时,布朗运动造成的位移大于沉降速率造成的位移,这时,扩散作用比自由沉降更重要。
在湍流气体中,例如近地面大气,湍流扰动比布朗运动造成的影响要大得多。这种湍流扰动并不取决于颗粒,而是取决于介质和气流。受到这种湍流的影响,不管其沉降速度如何,颗粒仍然可以传播数千公里。因此,在欧洲或大西洋彼岸发现了来自撒哈拉沙漠的风成气溶胶(见图1)。
4.4. 凝并过程
凝并过程是颗粒粘附或融合的过程。颗粒粒径越小,附着力越强。凝并过程涉及到作用范围非常小的分子间力,例如范德华力。凝并过程主要是由布朗运动引起的,因为布朗运动会导致粒子碰撞。因此,凝并过程会导致气溶胶中颗粒数量减少,同时会导致颗粒融合,粒径逐渐变大。这一过程中颗粒密度保持不变。凝并过程在颗粒数量较多时经常出现。当颗粒具有不同直径时,凝并过程发生更为频繁。凝并过程发生频率最低时,每立方厘米包含106个相同颗粒的气溶胶,其数值浓度减半需要 33 分钟,而当初始浓度为每立方厘米包含105 个相同颗粒时,气溶胶数值浓度减半则需要 5.5 小时。
由于凝并过程的存在,在没有持续产生新的细小颗粒的情况下,细小颗粒无法在气溶胶中持续存在。气溶胶的粒子数浓度越高,它们的寿命就越短。
5. 颗粒力学特性的影响
5.1. 颗粒采样
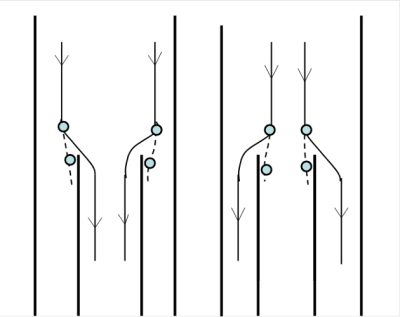
[来源:©J.Fontan]
当对气溶胶中的颗粒进行采样时,如果我们想要得到完整的粒径谱,就必须避免所采集颗粒的颗粒谱受上述现象的影响。然而,正如4.2节所述,惯性常用于对PM10和PM2.5进行采样,而在采集最细小的颗粒(纳米粒子)时,我们必须尽量减少布朗运动的影响。
因此,采集气溶胶中的颗粒需要谨慎操作,尽可能地避免颗粒力学性能上固有的不确定性。水汽或有机产物之类的气体可以在颗粒上凝结,采集颗粒时这些成分也会蒸发。因此,采集的颗粒应尽可能地与待分析的气溶胶中的颗粒性质保持一致(图5)。
5.2. 颗粒在呼吸系统中沉积
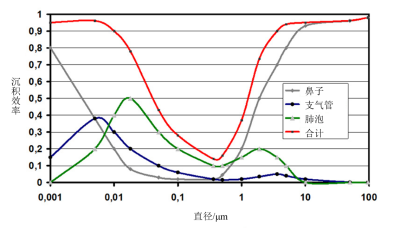
[来源:©J.Fontan]
当我们深入人体呼吸系统,可以把肺部看作是由众多圆柱体组成,这些圆柱体会分叉,最终形成以球体为示意图的细胞。根据这个模型,我们可以通过惯性、扩散和沉降来计算颗粒沉积量。大于10µm的大部分颗粒通常会沉积于鼻腔,并不会深入呼吸道。
图6显示了不同大小的颗粒在呼吸系统不同部位的滞留情况:
- 由于惯性,大颗粒多沉积于鼻咽段;最细小的颗粒(小于10-2µm)由于布朗运动也沉积于鼻腔。
- 小于5µm的颗粒会穿过支气管和肺部,部分会沉积在那里。
- 超过10µm的颗粒多沉积于鼻腔,并不会进入呼吸道、支气管和肺。
- 可吸入颗粒的直径小于10µm[4][5]。由于布朗运动,最细小的颗粒并不会沉积在肺泡中。
- 颗粒直径在1µm和1µm之间时,惯性就不会像大颗粒那样是主导因素了,而扩散还没有起作用。因此,这些细颗粒可以深入呼吸道内,但它们的滞留量还是最少的。上述内容强调了颗粒的力学特性与其在呼吸道中沉积量风险之间的关系[6]。
6. 颗粒的光学特性
探究颗粒的光学特性可以解决空气污染带来的能见度问题,并提供有关气溶胶的定性信息。颗粒的光学特性,尤其是通过卫星观测到的光学特性,可用于测量颗粒的直径和浓度[7]。
气溶胶颗粒可以对光造成漫反射。颗粒直径小于0.1µm时发生的散射称为瑞利散射(参见文章:天空的颜色)。瑞利散射的总辐射能量与R6/λ4成正比,其中R是假定的球形颗粒的半径,λ是辐射波长。短波辐射比长波辐射产生的总辐射能量大得多。相同能量情况下,蓝光的散射范围大约是红光的八倍。天空的蓝色、平静海面的蓝色,都是空气或水分子瑞利散射的结果。同样,香烟烟雾和由极细小颗粒组成的薄雾也呈蓝灰色。

[来源:拍摄者:Wolfgang Staudt(最初发布于Flickr),来自维基共享资源]
当粒径与波长接近或更大时,散射会偏向发生在入射光的方向。辐射波长对散射现象影响不大。由于散射作用,多云的天空或颗粒大于1µm的天空会变成白色。散射光不会随颗粒粒径的大小而变化。
颗粒大小对烟雾颜色、形态等具有影响,例如香烟烟雾。当香烟烟雾从鼻腔呼出后,烟雾的颜色会变得更白,这是因为呼吸道中的水汽使碳颗粒的直径变得更大了。这种光学特性的变化表明粒径会随空气湿度的变化而改变。这一点对于测量颗粒的质量浓度十分重要(参见文章:柴油发动机和木材燃烧)。大气气溶胶色彩的不同揭示了颗粒的不同来源。如果颗粒来自气固转变,则气溶胶呈现蓝色雾状。如果颗粒来自风蚀(例如欧洲上空来自撒哈拉沙漠的颗粒),气溶胶则呈白色(见第7.1)。
日出或日落时,太阳光呈红色(图7)。这是因为波长较短的蓝光被散射后逐渐消失,而红光波长较长,经过散射后仍然存在。当粒径与波长近似或更大时,散射会偏向发生在入射光的方向上。辐射波长对散射现象影响不大。由于散射作用,多云的天空或颗粒大于1µm的天空会变成白色。散射光不会随颗粒粒径的大小而变化。
气溶胶颗粒也是由可以吸收光的物质组成的,例如碳颗粒。在地面和大气的红外辐射中是十分普遍的现象[8]。
7. 颗粒光学特性的影响
7.1. 能见度
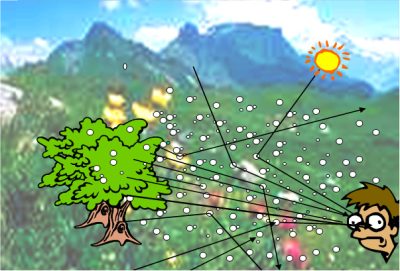
[来源:©J.Fontan]
在受到污染的空气中,由于颗粒对光的散射和吸收作用,观察者既可以接收部分来自观察物体的光线,也可以接收来自颗粒散射的光线(图8)。由于散射作用,物体与环境之间的反差降低。如果散射光特别强烈,则无法区分物体与周边环境。这种现象类似于水汽凝结成细小水滴时产生的雾气。

[来源:维基共享资源]
这种因空气污染导致的能见度问题在发展中国家的大城市十分普遍(见图9)。从飞行在大气边界层上方(海拔约1公里)或从山丘和山脉上空飞行也可以观察到城市能见度问题。在非洲萨赫勒地区,风蚀会在旱季将大量灰尘带入大气,导致能见度显著下降,能见度可降至100米左右,严重影响空中交通。
7.2. 对地面-大气辐射平衡的影响
气溶胶颗粒在大气辐射平衡中起着十分复杂的作用。颗粒能够扩散和吸收部分太阳光线,主要吸收太阳红外辐射。颗粒也可以将光线反射到太空,从而使地球降温。此外,颗粒还会吸收来自陆地的部分红外辐射,从而导致低层大气变暖并产生温室效应。这两种机制的综合效应取决于大气气溶胶的特性和地表土壤的特性及其对太阳辐射的反射系数(即反照率)。(参见文章:大气层和地球的气体包层)。这也是目前准确量化颗粒对地球辐射平衡影响的困难所在。一般认为,人类活动产生的颗粒会减缓温室气体导致的气温升高。
8. 主要知识点
- 空气中的颗粒是复杂混合物——气溶胶的组成部分之一。
- 颗粒的尺寸范围非常广泛,从几纳米的超细颗粒到几百微米大颗粒。
- 颗粒的物理特性(沉降、惯性、凝并)以及在采样系统和呼吸系统中的沉降能力随其大小而有很大不同。
- 超细颗粒一般不进行质量浓度的测量。
- 颗粒具有一定的光学特性。空气中大量颗粒的存在会影响空气透明度并降低能见度。
- 颗粒的辐射平衡十分复杂。颗粒能够吸收地球的部分红外辐射,从而导致温室效应和全球变暖。但是,颗粒也能将部分太阳辐射反射回太空,有助于地面冷却。
参考资料及说明
封面图片:开罗城市污染产生的烟雾 [图片来源:维基百科,作者:Sturm58] [GFDL (http://www.gnu.org/copyleft/fdl.html) or CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0)],来自维基共享资源]
[1] 对数是用加法代替乘法的数学运算:log(ab)=log(a)+log(b)。一般情况下,log10指的是十进制对数,log指的是Naperian对数,底数是无理数e=2.71828。
[2] Whitby K.T., Sverdrup G.M. Adv. About. Science Technology, 9, 477-525, 1980.
[3] Fuch N. The mechanics of aerosol, Mac Millan, New York 1964.
[4] Task group on lung dynamics. Health Phys. 12, 173, 1966.
[5] ICRP. Human respiratory tract model for radiological protection. Ann. ICRP 24, 1-3, 1994.
[6] 有关颗粒特性及其对健康影响的更多信息请参见:《颗粒与健康》一文。
[7] http://www.lmd.polytechnique.fr/~menut/documents/2016-LaMeteoSatAQ-REVetFIGS.pdf
[8] Renoux A. Boulaud D. Aerosols. Physics and metrology. Lavoisier TEC and Doc, 1998.
环境百科全书由环境和能源百科全书协会出版 (www.a3e.fr),该协会与格勒诺布尔阿尔卑斯大学和格勒诺布尔INP有合同关系,并由法国科学院赞助。
引用这篇文章: FONTAN Jacques (2024年3月13日), 空气污染颗粒究竟是什么?, 环境百科全书,咨询于 2025年4月2日 [在线ISSN 2555-0950]网址: https://www.encyclopedie-environnement.org/zh/air-zh/air-pollution-particles-what-are-they/.
环境百科全书中的文章是根据知识共享BY-NC-SA许可条款提供的,该许可授权复制的条件是:引用来源,不作商业使用,共享相同的初始条件,并且在每次重复使用或分发时复制知识共享BY-NC-SA许可声明。