云层中发生了什么?

雾、积雨云、卷云……云存在于任意不同高度的大气中,它们在形状、外观、温度和组成方面有各种各样的特点。世界气象组织出版了《云图》(Cloud Atlas),并在其中提出了区分与识别云和相关大气现象的参考系统。在本文中,我们将了解云的定义、形成、演变,以及对天气预报的意义。
1. 什么是云?
云由空气、水蒸气以及悬浮在大气中的液态或固态水粒子组成。这些名为水汽凝结体(hydrometeor)的粒子在大小、形状等方面千变万化,正是因为它们的存在,我们才可以看到云的存在(详见《天空的颜色》),并一览众多美不胜收的光学现象(详见《壮观的彩虹》、《大气晕》)。云中的凝结水质量相当可观,仅晴天的一朵积云就可容纳超50吨的水分。
同所有的大气颗粒一样,水汽凝结体一方面受到自身重量的影响,另一方面受到周围空气的摩擦。在无空气流动的情况下,所有的水汽凝结体都会下落,下落速度快慢不一,取决于粒子自身的大小、形状和密度。下表(表1)列出了水汽凝结体的典型的下落速度,其中小水滴的下落速度较慢,为每秒1 cm,而雨或冰雹的下落速度则较快,达到每秒几米。然而,大气从来都不是完全静止的,空气的运动也会扰乱水汽凝结体的运动。其中,湍流或弱对流上升气流之类的小位移足以使水滴和小晶体保持悬浮状态,这也解释了为什么云(如积云、卷云等)不会下落;而每秒几米的强劲上升气流则常见于雷暴,能够抬升大颗粒(如雨、雨夹雪),也是形成冰雹的必要条件。
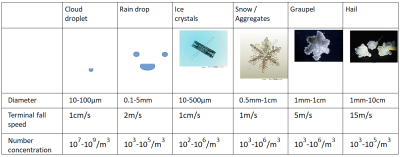
[表中图片来源:© 法国气象局;表:© Benoit Vié]
(Diameter 直径,Terminal fall speed 最终下落速度,Number concentration 密度大小,Cloud droplet 云滴,Rain drop 雨滴,Ice crystals 冰晶,Snow/Aggregates 雪/聚合物,Graupel 霰,Hail 冰雹)

水汽凝结体形状的多样性反映了它们的形成过程和特点。小云滴在高表面张力的作用下保持球形,而大的雨滴则会被压扁,这是因为雨滴越大,下落越快,空气对雨滴的摩擦力也越大,因而会使其变形(详见《移动物体的阻力》)。小晶体的形状则取决于其形成和发展过程中的大气条件,如温度和湿度等。如果这些条件保持不变,晶体通过气相沉积的方式生长,就可以保持其原本简单的形状。然而,对于体积更大、形成时间更久的晶体来说,当条件发生改变,或水汽凝结体之间发生碰撞,其形状就会变得不规则。
液态水汽凝结体最常在零上温度的云中观察到。然而,即使在较低的温度下,液滴也并不会轻易自行冻结。哪怕温度低至零下三十摄氏度,在云中仍有一定几率发现液态水。该现象被称为过冷液态水,且这种过冷状态并不稳定:液滴在接触到颗粒或冻结表面时很容易结冰。正是这种现象导致了冻雨的发生,以及飞机在飞行中的结冰(图1)。
同样地,虽然冰状水汽凝结体通常出现在零下温度的云层中,但它们落入零上温度的气团中时也不会立即融化。因此,水的液相和固相可以在云中共存并相互作用。正是这些相互作用导致了边缘水汽凝结体,如霰和冰雹的形成。
2. 云的形成条件
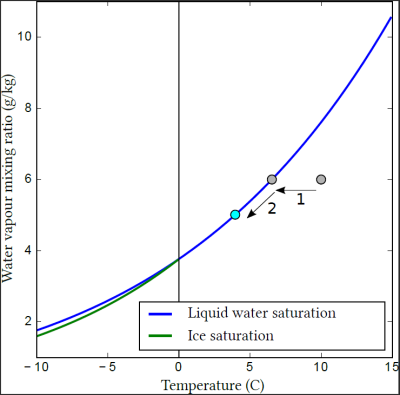
[来源:© Benoit Vié](Liquid water saturation 液态水饱和度,Ice saturation 冰饱和度,Temperature 温度,Water vapor mixing ratio 水汽混合比)
地球大气由干空气(氮气、氧气等)和肉眼无法识别的水蒸气混合而成。然而,大气所能容纳的水蒸气量是有限的。在液态水或冰的平坦表面,饱和蒸汽压,即大气中可容纳的最大蒸汽量,由温度和压强决定,并随温度升高而升高(图2)。
如图2所示,蓝色曲线下方的区域,空气中的水蒸气处于不饱和状态,可用的液态水(如雨滴或湖面)将会蒸发。相反,蓝色曲线上方的空气处于过饱和状态,水蒸气将会液化,附着在现有的液滴或表面上。刚好位于蓝色曲线上的点则代表空气中的水蒸气处于饱和状态,即使有液态水存在,也不会发生凝结或蒸发。
综上所述,当潮湿的空气团冷却时就会形成云。起初,这种冷却还不能形成云(图2,1)。只有当空气中的水蒸气达到饱和状态,并继续冷却,多余的水蒸气才会转化为水汽凝结体(图2,2)。

[来源:Pixabay,版权免费]
许多机制都可以冷却空气团并使之形成云,其中最简单的一种就是地表低温带动空气降温。然而,最为常见的一种则是大气中空气团的提升。由于大气压力随高度下降,气团将经历膨胀(expansion),并以对外做功的形式释放能量,从而导致自身的降温冷却。关于形成云的各种机制,以及通过这些机制产生的云的类型,可举例说明如下:
- 表面冷却和雾的形成
无论是温暖地区气团随气流的迁移,还是夜间地表的辐射冷却,都会导致地表温度低于近地面大气。这种情况下,热量就会从大气传递到地表。随着大气温度降低,水汽压达到饱和,空气中的水蒸气凝结形成露水。当冷却程度足够、大气条件有利(即风和湍流较小)时,雾(图3)可以通过上述冷却效应,自发地形成和发展。
- 地形抬升和焚风效应

[来源:Pierre Laïly, Flickr, Attribution-NonCommercial-NoDerivs 2.0 Generic, (CC BY-NC-ND 2)]
当潮湿的空气团被输送到山区时,地势将迫使其上升。空气团的抬升冷却导致迎风坡山脊上形成云和降水,气团随之变干。较干燥的空气越过山顶,沿背风坡下沉,受到大气压缩作用,温度重新升高。如果气团下降到比抬升前更低的高度,或如果降水极大减少了气团含水量,空气在下降过程中的升温幅度将大于抬升中的降温幅度,此时背风坡的温度将更高,称为焚风效应(foehn effect)(图4)。
- 低层大气辐合抬升(如海风)
在地形或海风风向与盛行风相反的情况下,大气环流可能会导致两个气团相辐合,在辐合区,暖湿气流被迫抬升,可能导致小型积云或雷暴的形成。
- 对流上升,积云和雷暴

[来源:Pixabay,版权免费]
太阳辐射会导致地表变暖,而高空仍然较冷,二者相互作用,可以强烈破坏大气的稳定,形成对流上升气流。如果大气低层水分充足,大气边界层的顶部将形成积云。当大气不稳定性较高时,例如春季,地表大量受热而高空仍然寒冷,这种机制可能导致雷暴的形成(详见《雷暴》)。同积云一样,雷暴的形成始于大气边界层的对流上升气流。温暖潮湿的空气上升冷却,并在凝结核(condensation nuclei)周围凝结形成云。水汽的凝结有助于加热气团,使之与环境之间形成密度差,从而提高其浮力,促进云的上升和发展。雷暴内部的垂直速度非常高,可以形成非常大的粒子,例如冰雹(图5)。
- 与全球大气环流的相互作用
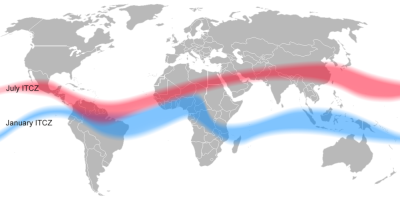
[来源:© Mats Hadlin]
在地球上,太阳辐射在热带辐合带(ITCZ,详见《信风的关键作用》)的增温效应最为显著(图6)。这种升温可产生非常强的对流上升气流,构成哈得莱环流(详见《大气环流》)背后的驱动力。地面低压的抽吸作用迫使来自热带的低层环流向热带辐合带靠拢。相对地,在对流层顶部(详见《大气和地球的气态外壳》),上升气流输送至此的空气被迫向周边辐散。此外,上升气流在引发强降水后会变得干燥,故该环流实际形成了一个充满干燥空气的下沉区,阻止了云的形成。这一原理解释了撒哈拉沙漠等部分热带地区的极端干旱。
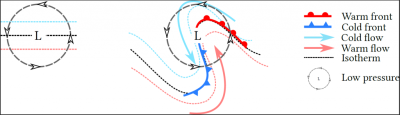
[来源:© Benoit Vié]
在温带地区,反气旋(表面高压区)和地表低压区(详见《热带气旋》)的交替影响着大气环流和云的产生。空气流动是从高到低的压力梯度引起的。因此,与热带辐合区相类似,温带低层的水平环流包括两种垂直运动:高空气流下沉,防止云的形成;低层气流上升,促进云的形成。
在低层,气团受到科里奥利力的影响,将围绕低压中心旋转。这种旋转发生在温度梯度较大的地区,如北半球南部,导致锋面的形成(图7):
- 冷锋,是冷空气向温暖地区移动形成的;
- 暖锋是暖流向寒冷地区移动,与冷空气相遇形成的。
两种情况下,密度较低的热空气的抬升均会导致云和降水的形成。
因此,云通常是大气环流和太阳辐射等外部作用力的结果。然而,云也可以对环境产生反馈。例如,云具有重要的辐射效应,可以改变大气的能量平衡。在白天,其反射和吸收部分太阳辐射,限制地表升温,增加自身热量;在夜间,其吸收地表发出的相当一部分红外辐射,并基于自身温度将辐射发回地表,从而极大地减弱了地表的辐射冷却效应。该现象的一个典型例子是,高空云层的存在可以阻止地面雾的形成。
对流云也能直接影响大气动力学。强烈的上升和下沉气流导致大量气团发生空间置换,在温度、湿度等方面形成强烈的局部对比。就像海浪一样,重力波可以在两个密度不同的气团的中间界面上形成。这些波在大气中传播,并可能在不稳定的气团中催生新的对流单体。另一种机制与雷暴中降水的蒸发冷却有关。蒸发效应能够形成一个巨大的冷空气团,称为冷池(cold pool)。当冷池与温暖、潮湿的低空气流相遇,将会引发进一步的对流抬升。该机制非常重要,例如,热带地区的飑线作为一种准静止对流系统,却能够在地中海地区引发强降雨和毁灭性的山洪暴发(详见视频)。
3. 云中水滴的形成
在自由大气中,蒸汽交换不是发生在平坦的表面上,而是发生在液滴上。可以认为液滴是球形的一级近似。要确定液滴附近的饱和蒸汽压,必须考虑两方面影响。
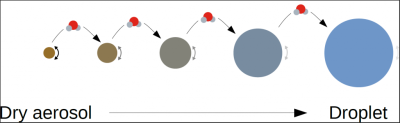
第一种是开尔文效应(Kelvin effect),与液滴的表面张力有关。由于液滴很小,冷凝过程又很复杂,开尔文效应因此变得更加重要。开尔文效应指的是纯水滴表面的平衡饱和度与曲率半径成反比,由此可得纯水滴的形成需要百分之几百的过饱和。这就解释了为什么大气中的雨滴不是自行凝结的,而是由名为气溶胶(aerosol)的大气微粒形成的,这种微粒的大小从几纳米到几微米不等。气溶胶既可以从自然界获得(如海盐、有机气溶胶等),也可以通过人为活动产生(如污染产生的黑碳)。
其中一些气溶胶可溶于水,可作为形成液滴的核。此时就需要考虑决定液滴表面平衡饱和的第二个重要效应——拉乌尔效应(Raoult effect),它与水溶液中促进凝结的组分有关。可溶性组分的浓度越高,这种效应越强。对于给定的初始气溶胶,拉乌尔效应取决于凝结水的体积,即液滴半径的立方(图8)。
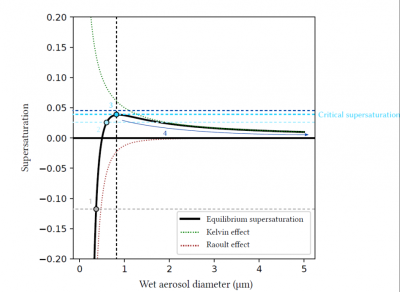
在干燥的气溶胶或捕获了很少水分的气溶胶中,拉乌尔效应占主导地位,此时平衡过饱和度(equilibrium supersaturation)可能为负。这些颗粒可以捕获水蒸气,并在大气没有达到饱和的情况下增长。随着颗粒不断储水增长,拉乌尔效应会逐渐减弱,直至弱于开尔文效应。这解释了为什么平衡过饱和度在某些直径下可以是正的。最后,当液滴变得足够大,通常是直径几微米时,因为其主要由水组成,故拉乌尔效应和开尔文效应均可以忽略不计,整体达到接近于0的过饱和平衡。
图9描述了一个平衡过饱和的例子,是某一气溶胶湿直径的函数。曲线的拐点取决于气溶胶的成分及干燥状态的直径,通过该拐点可以确定气溶胶的临界过饱和度,即气溶胶即将被激活成小液滴的环境过饱和度。
4. 云中小冰晶的形成
[来源:!;原作者:Pre2grkVector:JoKalliauer/CC BY-SA (https://creativecommons.org/licenses/by-sa/4.0)]
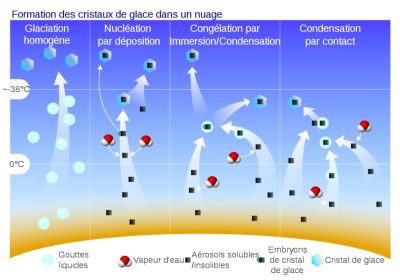
(Formation des cristaux de glace dans un nuage 云中形成冰晶,Glaciation homogène 成分均匀的冰期,Nucléation par deposition 沉积成核,Congélation par Immersion/Condensation通过浸泡/冷凝冷冻,Condensation par contact 接触冷凝,Gouttes liquids 滴液,Vapeur d’eau 水蒸气,Aérosols solubles/insolibles 气溶胶可溶性/不可溶性,Embryons de cristal de glace 冰晶胚胎,cristal de glace冰晶)
同样地,一些被称为冰核(ice nuclei)的气溶胶会促进冰晶(ice crystal)的形成。冰核的作用方式并不像凝结核那样出名。其启动晶体形成的能力与自身表面特征有关。在冰核表面某些地方,存在着有利于冰晶发育的几何构型,被称为活性位点(active site),晶胚就是在活性位点上形成的。活性位点的表面密度取决于气溶胶的化学成分,诸多实验都试图预测这些气溶胶演变成晶体的成核过程。不同于凝结核,冰核没有单一的作用方式,它们可以通过蒸汽凝华直接在活性部位形成晶体,也可以通过接触冻结过冷的液滴(图10)。冰核演变成小晶体的成核过程称为冰晶的初生(primary production)。
关于冰核的作用方式,目前学界仍存在争论:
- “随机”假说强调活性部位激活的随机性。根据此假设,冰核的活性取决于其暴露在有利的热力学条件下的时间长短。
- 相反,“单一”假说为每个活性位点指定了一个激活温度,该温度取决于其几何构型。因此,只要温度达到其最有利的活性位点的激活温度,每个冰核都可以通过成核过程形成冰晶,该过程因而与时间无关。上述两种成核过程究竟孰真孰假?要回答这一问题,必须且只能在实验室中精确控制变量、反复进行成核实验。出于实验上的困难,我们暂时无法对成核的作用方式下定论。
然而,对冰核和冰晶的联合观测显示,二者之间始终存在巨大的数量差异,冰晶的数量往往远远超过冰核的数量。因此,小冰晶的形成还存在其他机制,称为冰的次生(second ice production)。初生与次生是目前已知的两种晶体形成机制。
- 当温度极低时(该阈值取决于液滴大小,一般为-35°C),高空中集中出现的大量小晶体可能是水滴冻结(图9)形成的。
- 而当温度处于-3°C和-8°C之间时,Hallett-Mossop机制则更为活跃:冰粒、雪或雨夹雪在收集和冻结过冷的液态水滴时,有时会破裂并发射出冰晶碎片。该机制很大程度上增加了小晶体的浓度。
此外,还存在其他一些难以通过观测来量化的成核机制。例如,冰晶和雪花与密集的冰粒碰撞时会发生碎裂,较大的过冷雨滴冻结时也会溅射碎冰。对于处于冰相或混合相的云而言,其生成和演变过程均十分复杂,难以预测。
5. 云的演变和降水的形成
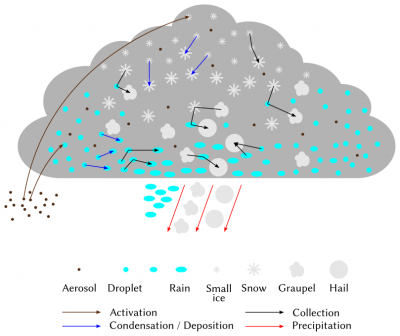
[来源:© Benoit Vié](Aerosol 气凝胶,Droplet 液滴,Rain 雨水,Small ice 小冰晶,Snow 雪,Graupel 霰,Hail 冰雹,Activation 活化,Collection 聚集,Condensation/Deposition 冷凝/凝华,Precipitation 降水)
云层形成后,水分子和水蒸气之间会发生一系列相互作用,从而推动其演变(图11)。其中涉及的一组过程是水蒸气和水滴(晶体)之间通过凝结(凝华)和蒸发(升华)进行水的交换。如果气团在云形成后继续上升和冷却,大气中多余的水蒸气将继续凝结在现有的液滴上并不断增长。相反,如果气团的相对湿度降低,液滴就会蒸发,直到恢复饱和状态。需要注意的是,零下温度下,冰面饱和蒸汽压低于水面饱和蒸汽压。因此,在零下温度的云中,如有水滴和小晶体共同存在,则晶体更倾向于通过凝华来捕获水蒸气,损失的蒸汽压则由水滴蒸发来补充。这一现象称为伯杰隆效应(the Bergeron effect),可以导致空腔云(cavum-type cloud)的形成(图12)。

[来源:H. Raab (User:Vesta) / CC BY-SA(https://creativecommons.org/licenses/by-sa/3.0)]
另一组过程涉及不同的水汽凝结体之间的碰撞和凝聚。大气中的所有水汽凝结体都在运动,粒子之间的碰撞能够改变水汽凝结体的大小和类型,以及在水的相变过程中的温度,从而直接影响云的演变。在每次碰撞中,水汽凝结体可能会合并,也可能不合并。例如,两个小水滴很容易合并成一个更大的水滴,但冰粒子之间的冲击可能会导致水汽凝结体的破碎。此外,碰撞也可能引发相变。例如,过冷的液态水与冰晶接触时就会冻结。
在云的形成过程中,只要云是由大小差异不大的小水滴或晶体形成的,云中稀少且微弱的湍流和空气运动就会引发颗粒之间的碰撞,逐渐形成较大的水汽凝结体。当不同大小的凝结体以不同的下降速率同时存在于云中时,较大的颗粒就会与许多较小的颗粒接触。上述过程即为降水形成的主要过程。
云的初始成分对其演变和降水的形成也起到决定性作用。在由大量微小悬浮液滴组成的云中,收集-凝聚过程会非常微弱,不足以形成降水。相反,如果云起初由少量大小不一的大液滴组成,那么雨的形成就会更快。这也解释了为什么气溶胶不仅决定水汽凝结体的数量和大小,而且会对其生命周期产生重大影响。综上所述,从雾到对流系统,气溶胶对所有的云都有着显著的影响。
6. 云在数值天气预测模型中的表现
在数值天气和气候预测模型中(详见《天气预报模型》和《气候模型》),不可能单独预测每个水汽凝结体的演变。因此,有必要在每个模型网格中更综合地表示水汽凝结体的特征。微物理参数(microphysical parametrisations),又称云物理方案(cloud scheme),可以实现这一目标。
最精确的微物理方案根据水汽凝结体的类型(如水滴、晶体等)和大小,将水汽凝结体分为非常多的类别,并预测每类水汽凝结体在云中的数量。该方案精确度极高,例如,方案中的一个变量为直径在1 mm和1.1 mm之间的雨滴的数量。因此,这些方案能够准确地表示云的组成,以及云随时间演变的生命周期,从液滴附着于气溶胶形成云,到降水形成,再到云的消散。然而,由于所需变量太多,对于实际的天气预报来说过于昂贵,因此这类方案只能用于学术目的。然而,即便如此精确,其中的变量也会取近似值,因为方案中仍然存在各种误差:如与空间和时间离散化相关的误差源,分类细度选择方面的敏感性,以及大多数过程描述中的不确定性边际(margin of uncertainty)。事实上,仅是定义形状不规则的水汽凝结体的尺寸就已经很困难了。然而,在尺寸固定的前提下,其特征(表面积、密度、质量、下降速度等)仍会产生很大的变化,因此,仅按凝结体尺寸分类是远远不够的。冰晶类的水汽凝结体甚至更加微妙、更加难以描述。
更简单的微物理方案则仅按照大类划分水汽凝结体(如云滴、雨、雪),并假设每种类型的水汽凝结体的空间分布遵循预定义的概率规律,自由度很小。最简单的方案称为单矩方案,仅包含一个自由度,通常只能预测每类水汽凝结体的总质量。因此,它们不能准确地描述云的组成以及气溶胶对云的影响。要达成上述目的,需要更复杂的方案,其中包含两个及以上的自由度。除了质量外,该方案通常还可以描述每种类型的水汽凝结体的数量,甚至描述反射率或其他特征。此外,模型还将分别记录所有微物理过程(包括一种或多种水汽凝结体间的相互作用)对模型预测变量的影响。例如,在液滴自聚集过程中,液滴总质量不变,但总数减少;又如,可以根据所有其他水汽凝结体的积聚,来描述冰雹的发展过程。
相比于精确方案,以上方案的成本要低得多,更常用于天气和气候预测模型。例如,法国气象局的高分辨率天气预报模型(AROME)就是使用一种单矩方案,向法国大都市和海外部门提供长达36小时的详细预报。为了改进云的参数化方案,并将气溶胶-云的相互作用纳入考量,法国气象局现正在测试一种两矩方案,其中新增了水汽凝结体和气溶胶数量浓度预测值两个变量。
7. 总结
- 云是由水汽凝结体、液态水粒子(云滴和雨滴)和/或固态水粒子(小冰晶、雪、冰雹等)组成的。
- 大气中可容纳的水蒸气量由空气的温度和压强决定,并随着温度的升高而减少。因此,当温暖、潮湿的空气冷却时,就会形成云。
- 空气的冷却可能是由辐射引起的,也可能是由于空气的抬升导致压力降低引起的。
- 云的形成一般由大气环流驱动,大气环流能够催生上升气流,是推动云形成的主要原因,但某些云系统(如气旋、雷暴等)中的强烈环流也会对大气环流产生反作用。
- 大气中的液滴不是自行形成的,而是在被称为气溶胶的悬浮颗粒上形成的,这些颗粒有助于水蒸气凝结。因此,大气中存在的气溶胶的数量和类型可以深刻影响云的形成和生命周期。
- 最后,云的演变既受热力学条件改变(如辐射交换、水相变化过程中的潜热释放)的控制,也受水汽凝结体之间多种相互作用的影响。
环境百科全书由环境和能源百科全书协会出版 (www.a3e.fr),该协会与格勒诺布尔阿尔卑斯大学和格勒诺布尔INP有合同关系,并由法国科学院赞助。
引用这篇文章: VIE Benoit (2024年2月24日), 云层中发生了什么?, 环境百科全书,咨询于 2025年3月7日 [在线ISSN 2555-0950]网址: https://www.encyclopedie-environnement.org/zh/air-zh/whats-happening-in-the-clouds/.
环境百科全书中的文章是根据知识共享BY-NC-SA许可条款提供的,该许可授权复制的条件是:引用来源,不作商业使用,共享相同的初始条件,并且在每次重复使用或分发时复制知识共享BY-NC-SA许可声明。






