海洋环境

地球表面三分之二以上是海洋。在阳光的照射下,海水的温度会因位置和深度而异。海水的成分和盐度虽在不同海域之间有所差异,在同一海域中则非常稳定。海洋环境可谓生命的摇篮。在既非古人所认为的平坦、亦非真正球形的海水表面下,大量的生物生活在与陆地截然不同的环境中,构成了惊人的生物多样性。
1. 海洋的起源和范围
在地球的缓慢演化过程中,板块构造不断改变着大陆和海洋的位置。在大约3亿年前的盘古大陆时代,这片整体大陆的周围只有一个大洋[1]。今天,根据国际水文组织(IHO)的分类,有三个大洋。最大的是太平洋,其表面积约为整个海洋的一半、整个地球表面的三分之一。正因为太平洋面积之大,人们把它的中央经线选定为国际日期变更线。第二大的是大西洋,约占海洋总面积的30%。它具有全世界最大的淡水汇入量,受纳了来自亚马逊河、刚果河和圣劳伦斯河等大河的水流。印度洋的面积第三大,约占海洋总面积的20%。它基本位于南半球,被亚洲、非洲和澳洲大陆所包围。
尽管IHO将海洋分为三大洋,但人们更习惯将海洋分为五大洋。南面分化出南冰洋(或南大洋),由南极大陆海岸线延伸至南纬约60度,其面积约占海洋总面积的6%。北面分化出的北冰洋,与西伯利亚、斯堪的纳维亚、格陵兰和北美大陆相邻,其面积约占海洋总面积的4%。北冰洋深度较浅,一部分由浮冰覆盖着。这五大洋的表面积占全球总表面积的71%。
海域则是指为相对较小、相对独立的海洋子域。这种独立性通常归因于地理上的隔离。地中海就是如此,它只通过极其狭窄的直布罗陀海峡与大西洋相连,以至于大西洋的潮汐在地中海海岸已衰减得微乎其微(见“潮汐”)。相反,英吉利海峡则大部分与大西洋贯通。来自大西洋的高潮直接抵达英吉利海峡,减缓于英国和法国之间的加莱海峡减缓,进入北海之后,在丹麦和瑞典之间的狭窄通道进一步衰减,最终悄无声息地到达波罗的海。在其它情况下,例如加勒比海,即使地理上确实有分隔(从委内瑞拉海岸到佛罗里达半岛的一长串岛屿作为分隔标志),但其海域的概念更多地是脱胎于历史因素(沿岸国家的沿革和征服史)甚于地理因素。
2. 海水的密度、盐度和温度
在压力101.3kPa、温度3.98°C、盐度为0的参考条件下,水的密度正好是103 kg/m3,由此引申出千克的第一个定义,即一升淡水的最大质量。海水的密度主要随温度和盐度而变化,随压力的变化则较小,因而人们往往认为这种液体是不可压缩的。
盐度是指海水中盐的质量分数,或单位质量海水中所含盐的质量比率,用g/kg(每千克海水中的含盐量)表示。在湖泊和河流中,盐度几乎为零,很少超过几g/kg。在海洋中平均盐度可达35g/kg,有时超过50g/kg。黑海的盐度为12g/kg。死海的盐度接近275g/kg,几乎任何动植物都无法在如此高的盐度下生存。
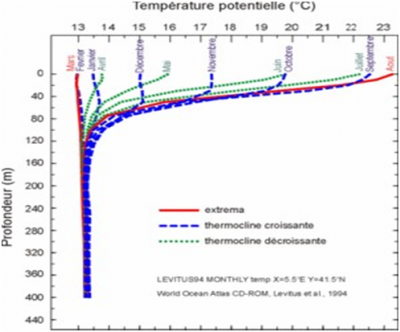
在海洋中,海水的温度[2]在2到30°C之间变化。而在地中海,海水温度的变化范围较小,在13到24°C之间,如图1所示。
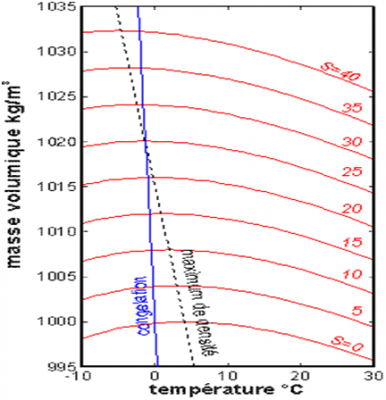
(Masse volumique 密度,Temperature 温度,S为盐度)
海水密度同时随温度和盐度变化,如图2所示。一般盐度越高密度越大。对于淡水,密度在约3.98°C时最大,在13°C至30°C之间随温度升高而减小。此外,在夏季,最温暖海域的表层水温可达26°C至30°C,这往往会导致气旋(见“热带气旋:发展和组织”)。
在海洋环境的上层,表层水受到太阳辐射的照射,通过与大气的对流和传导进行持续的热交换。在波浪和湍流的搅动下,在最初的几十米(0到-50米之间),温度会趋于均匀。相反,在很深的地方(低于-120米),热交换几乎仅限于纯传导,并变得非常微弱,此时海洋环境趋于静止,即便深水缓流和表层洋流会略微放大深层水的表观导热系数。
这两层之间的区域(-50到-120米之间)通常称为温跃层,其上下两侧的温度跨度达到约10°C。由于阳光的影响,温跃层上方的水温具有显著的季节变化特征,而深层的水温则没有任何变化。由图1可见,随着阳光的增加,地中海的温跃层在春天建立,8月份达到最大温差10°C左右,秋季和初冬温差渐小。到2、3月份,温跃层消失,水温在整个深度上几乎不变,在13℃和13.5℃之间。值得注意的是,温跃层上下方的温度变化与所在深度有关,上方受热的流体层略微受到瑞利-贝纳特对流不稳定性的影响,这使得表层水下降以补偿深层水的上升。
这种垂直运动虽然非常缓慢,但由表层水下沉所带来的氧气对深层海洋生物至关重要。相应的上升流通过将营养物质从海底带到表层,对表层的海洋生物也大有裨益。在地中海,这种垂直对流现象主要位于利翁湾,使其生物活动异常繁茂。然而,这种对流只有在满足必要条件时才会发生。黑海就不具备这样的条件,在密度上始终保持恒定的分层,表层氧气无法渗透超过200米的深度,只有非常特定的物种才能生活如此的缺氧环境中。在全球范围的温盐环流中,类似的对流现象在深处将世界大洋融合在一起。
3. 海水的成分
海水的化学成分并不简单。大多数化学元素以阴离子、阳离子和分子的复杂混合物形式存在于海水溶液中。离子并不仅限于氯离子和钠离子,尽管这两种元素是海盐(氯化钠)的主要成分。表1按重要性排序列出了盐度为35g/kg的普通海水中含量前五位的阴离子和阳离子。碰巧的是,所有这些离子浓度之间的比率在不同的海洋之间变化很小[3],因此我们只需测量其中一种成分的含量,就可大致推断出总盐度。
这些元素有着不同的起源。一些离子来自于大陆岩石,经河流溶蚀后流入海洋。这些离子在海洋中停留了漫长的岁月,随着水分的蒸发而不断变浓。很大一部分阳离子来自原始的海底。而氯离子的来源往往归因于火山喷发的氯化氢气体溶入海水之中。
表1. 盐度为35g/kg的典型海水中的主要离子浓度
除了水和盐,还有各种低浓度的分子,如硼酸(0.0198g/kg)、二氧化碳(0.0004g/kg),还有氮气和氧气。值得注意的是,海水中的二氧化碳含量比空气中的大得多,大约是空气中的60倍,而这个数值并不是海洋吸收二氧化碳含量的上限。目前,一个备受争议的问题是:是否能通过封存海洋中的二氧化碳以减少大气中二氧化碳这种温室气体的含量。虽然对可能发生的反应存在不确定性,例如海水酸碱度[4]或碳循环的显著变化,一些人建议在排放源附近捕集二氧化碳,并将其直接注入海洋深处。
4. 大海的起伏不平
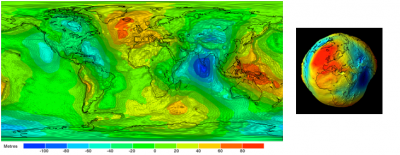
因为质量在地幔中不是均匀分布的,所以地球表面的重力分布也不均匀。仅这一个因素就意味着海洋高度必然会因局部重力而变化,而不是绝对不变。在重力最小的地方高度最大,反之亦然,二者的乘积为常数。因此,海洋的平均自由表面无法与球体表面完全一致。然而,由于所有的高度都是在平均海平面基础上定义和测量的,因此有必要事先明确这个平均海平面代表什么[5]。海洋学家和地球物理学家选择与平均海平面最吻合的重力场(或重力)等势面作为参考,如图3所示。这个特殊表面被称为大地水准面,突显了实际海洋表面与理想球体(均匀重力场)的偏离程度。这个凹凸不平的表面反映了地球表面(大陆或海洋表面)重力的变化[6]。
5. 海洋环境中令人惊叹的生物多样性
在46亿年前形成的地球上,生命在大约38亿年前就出现在海洋中。而直到大约4亿年前,生命才征服了陆地。海洋孕育出了极其丰富多样的生命。在这里,光线只能穿透海洋表层。海洋生命逐渐适应了这种海洋环境的特殊条件。人们认识到,只有少数物种能够在陆地上穿行并使它们与众不同。这意味着,我们星球上的大多数生物多样性都是由海洋环境贡献。通常估计海洋环境中的物种数量在500万至1000万之间,但这些物种中的大多数,特别是深海物种,仍然未知。相比之下,生活在陆地上的物种数量约为130万,其中昆虫的数量约为85万种(阅读“什么是生物多样性?”)。
参考资料及说明
[1] 这块被称为盘古大陆的单一大陆可以追溯到古生代(距今5亿到2.5亿年前,或称Ma),这个地质时代之前是前寒武纪(2500到500 Ma),之后是中生代(250到65 Ma)、新生代(65到1.65 Ma)和现在的第四纪的地质时代。
[2] S. Levitus and T.P. Boyer, 1994, World Ocean Atlas, Vol. 4, Temperature, NOAA
[3] 这一经验性质被称为迪特马尔定律(Dittmar’s Law),以纪念苏格兰化学家威廉·迪特马尔(1859-1951),在1872年至1876年的挑战者号海洋考察期间,他从所采集的样本中分析得出这一定律。
[4] 首字母缩略词pH代表氢和浓度。该值测量溶液中氢离子(H+)的活度,衡量溶液的酸碱度。因此,在25℃的水体基质中,酸性溶液的pH值低于7,中性溶液的pH值等于7,碱性溶液的pH值高于7。
[5] 重力,或称为单位质量的重量,源于势能。与所有具有该属性的矢量一样,重力可以用与其正交的等位面来表示。就像地图上的等高线,表面越紧密,重力越高;表面越稀疏,重力越低。广义认为,大地水准面的凸起对应重力最小值,凹陷对应重力最大值。
[6] G. Balmino, F. Perosanz, R. Rummel, N. Sneeuw and H. Sunkel, Champ, GRACE and GOCE: mission concepts and simulations, Int. Gravity Commission and Int. Geoid Commission, N°2, Trieste, 1999, vol. 40, No. 3-4, pp. 309-319.
环境百科全书由环境和能源百科全书协会出版 (www.a3e.fr),该协会与格勒诺布尔阿尔卑斯大学和格勒诺布尔INP有合同关系,并由法国科学院赞助。
引用这篇文章: MOREAU René (2024年3月9日), 海洋环境, 环境百科全书,咨询于 2025年3月14日 [在线ISSN 2555-0950]网址: https://www.encyclopedie-environnement.org/zh/eau-zh/marine-environment/.
环境百科全书中的文章是根据知识共享BY-NC-SA许可条款提供的,该许可授权复制的条件是:引用来源,不作商业使用,共享相同的初始条件,并且在每次重复使用或分发时复制知识共享BY-NC-SA许可声明。