饮用水生产是一个重要的公共卫生议题

饮用水生产和供应是一个关键的公共卫生话题。自19世纪末以来,从微生物学的发现到超微量化学分析,取得了长足的进展。这是公共卫生不断改善的主要原因之一。平均而言,每个法国人每天消耗150升饮用水,但源头取水量还要高出50%。本文首先介绍饮用水生产技术,然后总结饮用水法规以及所涉及的风险。这些风险源于饮用水中的微生物(如隐孢子虫、蓝藻毒素等)和化学物质(如农药代谢物、药物残留物等)。饮用水处理对这些风险进行了全面的但不完全彻底的控制。本文还总结了气候变化的预期影响,其中一些已经得到观察。这些影响包括未来获取优质水资源的困难(河流径流量减少,地下含水层水位降低)(污染物再迁移,稀释效应减弱)。
专门用于人们消费的水,也就是饮用水,根据法规的定义“不含有可能对人体健康构成潜在危险的微生物、寄生虫或任何其他物质,并符合一定数量的质量限值和参考值。”。因此,饮用水通常是优质水。每一个法国人平均每天消耗的150升优质水,其中只有10%用于饮用和烹饪。相应的地下水和地表水取水量占全国总需求的18%。如果排除用于电力生产的取水量,其占比则接近42%。因此,考虑到气候变化对水资源的可利用性以及水质的预期影响,这一区域的风险会因此高得令人担忧。
1. 饮用水生产和供应:一个重要的公共健康议题
1.1. 病原体的发现
随着欧洲霍乱和伤寒疫情与污水污染之间关系的确定,才产生了与水相关的健康风险的认识。在法国,这些观测结果促使了在1900年的第一次部长级通告的发布,该通告宣称,化学分析(当时的化学分析非常简化)已不足以评估水的安全性,还需要进行微生物分析,但没有说明具体方法。第一次对相关法规的详细阐述是在1962年,这项法规的实施引出一个重要的但难以验证的水质要求:“不含任何致病性微生物”。在控制方面,若水中不含有所谓的“测试”细菌,则被认为可以代表水中存在令人恐惧的已知病原体(如沙门氏菌和志贺氏菌)的风险极低。
现在已经证明了“不含测试细菌”这一健康目标的有效性。这一健康目标可能对20世纪一些在公共健康方面所取得的巨大进步起到了重要作用。19世纪末大流行病肆虐,自那时起,人们确实在强调水过滤带来的好处。在20世纪初,慢滤和消毒(漂白、臭氧化、碘氧化)工艺得以发展,然后是使用凝聚剂进行澄清的工艺。该混凝过程包括两个步骤,首先是在待处理水中投加一种(或多种)化学试剂并快速混合,使水中的胶体(非常细小)颗粒脱稳,然后在缓慢搅拌(絮凝、混凝)的条件下使脱稳颗粒物聚集,目的是使这些颗粒物更容易通过沉淀(或过滤)去除。在20世纪六七十年代,饮用水生产的水处理过程仍然非常简化。地下水只是简单的用泵取水和氯化(或“漂白”),地表水则是根据经典的“预氯化,然后澄清(混凝、絮凝、沉淀、砂滤),最后氯化消毒”的方案进行处理。
1.2. 有毒化学品检测的持续进展
多数的物质来说,其所带来的风险主要是长期的。相关法规现已建立起来,并采用了最高允许浓度的理念,且得到了足够的重视。最近几十年,许多化学品在天然水体中被检出,其浓度水平也被测定,毒理学和流行病学的发展使得评估这些化学品对健康的影响成为可能。这些新污染物大多数是天然衍生化合物(肥料、金属等)以及新的合成化学品(杀虫剂、药品、化妆品等),其被检出是其大量生产和广泛使用的结果。其中一些污染物长期存在于饮用水中,过去其在饮用水中的浓度有时甚至高于目前的浓度。然而,过去的法规并未一直将其纳入其中,要么是因为其毒性未知而被认为是安全的,要么是因为对很多物质而言其存在的浓度低于仪器检测限而不能分析出来(例如三氯甲烷(THMs):用氯气或漂白剂消毒时产生的副产物,这类消毒副产物是由氧化剂”氯”与水中存在的溶解性有机物(天然的或人工合成的)之间发生化学反应的产物;例如还有溴酸盐、材料单体、天然激素等)。对于其中大多数的物质来说,其所带来的风险主要是长期的。相关法规现已建立起来,并采用了最高允许浓度的理念,且得到了足够的重视。
由于无法完全或高效地阻止有毒化学品进入水体,而且其带来的风险永远不会是零,故如今的水处理系统必须在其设计或改造过程中进行调整。上面提到的第一个简单工艺已经在很长一段时间无法胜任水处理需求了,目前已加入特定的处理过程专门用于消除存在于水中的化学物质(铁、锰、硝酸盐等)(更确切地说是针对地下水)和/或深度处理(通常针对地表水)以消除业已存在的或意外事件带来的污染。
因此,近几十年来涌现了许多先进的水处理工艺(见§3)。20世纪80年代开始使用的膜工艺,无疑是饮用水生产领域最重要的技术飞跃。膜工艺能够使自上世纪90年代以来一直在寻求的“无氯水”的概念的实现成为可能吗?在如今的绝大多数情况下,这仍然是不可想象的,主要原因是处理后的饮用水需要在管网中进行输配,而输配过程对微生物污染的发生仍然非常敏感。
2. 饮用水需要什么?从哪里来?
世界上约有10%(7亿)的居民无法获得水质足够好的饮用水(90年代末则超过30亿)。由于人口增长和全球变暖,全世界对饮用水的需求将越来越重要,特别是对于那些人均可利用淡水资源不足1000m3的国家将是一个相当大的挑战[1](见水资源短缺风险?)。法国每位居民拥有2600m3的可利用淡水资源,但存在很大的地理和季节差异。这相当于1700亿至1750亿立方米,其中有54亿立方米(5.4km3)被用于生产饮用水,其中略低于2/3是地下水,其它则是地表水。

[图片来源:宝琳·努齐尔 – 环境观察站,波伊图-查伦特斯]
这54亿立方米(5.4km3)的淡水满足了6700万居民的饮用水需求,相当于平均每人每年80.6立方米(或每天221升)。2012年,平均饮用水消耗量为54 立方米/人/年。城镇的消耗量高于农村。饮用水平均消耗量在20世纪90年代达到峰值,从那时起开始逐渐下降,主要是因为关心和为了降低水费,但也有用水行为发生变化的原因。水资源使用量和家庭用水量(在消费者水龙头处测量的用水量)之间存在差异(译者注:即产销差),有几个原因。据估算,发生在管网输配水系统中的水损耗量为每个居民每年约15.6立方米,这一数值在过去约15年相对比较稳定。输配水系统是由一整套部件和设施(管道、水箱/水池等)构成,用来将饮用水厂出水(泵压水)至消耗者的水龙头(分配水)。而(发生在生产过程中的)其他损耗以及(消耗饮用水的)第三产业、公共部门和工业部门的消耗可以解释除管道漏损之外的产销差。
在未来的几十年里饮用水用水量将会怎么样?在确定和实施气候变化适应计划之前很难预测。一些研究[2]预测由气候变化所导致的法国平均气温升高每增加一度就会增加约1.5%至2%的用水量。然而,与2017年6月(例如发生在新阿基坦)的“热浪”期间记录的用水量增长相比,这些数字似乎很低。应提供办法来限制这一增长。
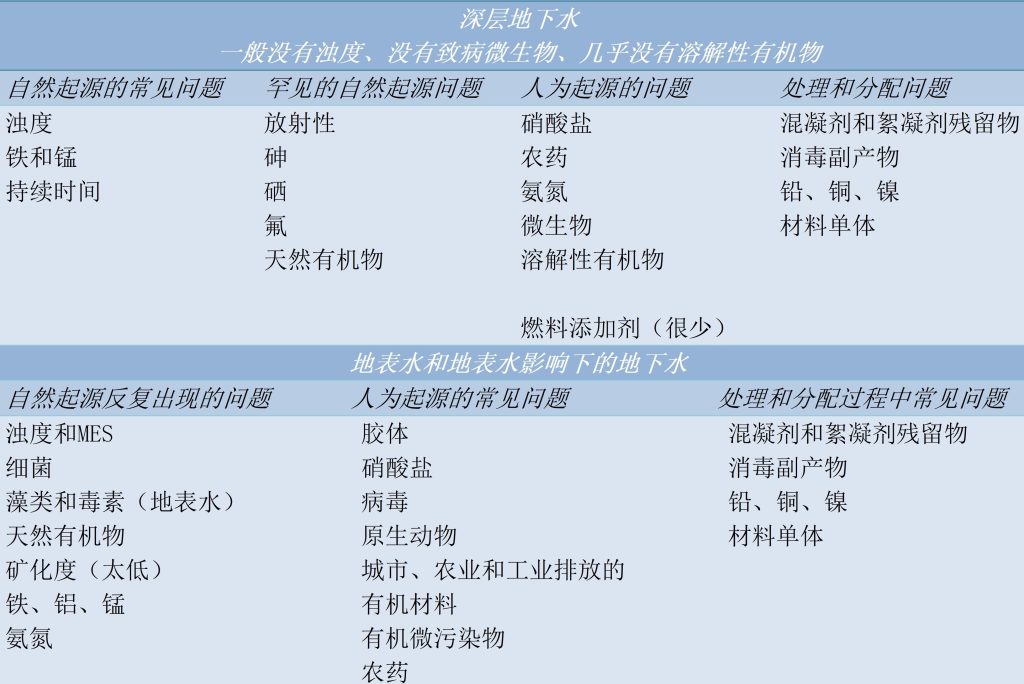
用于生产饮用水的水源水水质因其中所含物质(天然源和人为源;见表)类型的不同而差别很大。我们确实可以在水源水中找到:
- 细菌、病毒、寄生性原生动物、浮游植物等生物类成分;
- 对健康没有明显影响或有间接影响的矿物类成分,如浊度、颜色、矿化物质;
- 某些过渡金属、氨和某些溶解气体;
- 对健康有显著影响的矿物类成分,如重金属、氧化形态的氮、锑、砷、钡、硼、氟、硒等;
- 以综合指标表征的有机物类成分:总有机碳(TOC)表征那些能够被热氧化或光化学氧化的水中所有含碳类有机物质浓度;高锰酸盐指数(高锰酸钾作为氧化剂时的可氧化性)则表征水中含碳类有机物中能够被化学热氧化的部分;
- 早已发现的有机微污染物(杀虫剂、氯代溶剂、碳氢化合物、多氯联苯、洗涤剂)和新近出现的微污染物(新污染物)(内分泌干扰物、药物残留、化妆品等);
- 具有放射性的成分。
此外,还会通过处理和输配水过程引入新的杂质,如水处理药剂的残留(铝、铁)、消毒副产品(三卤甲烷、溴酸盐、亚氯酸盐)、铅以及与水接触的聚合物材料单体。这些杂质的存在会导致水处理过程中需要解决不同的问题。
水处理过程中遇到的、直接或间接地与水源水质相关的主要问题有:
3. 法国饮用水的生产和输配
在法国,饮用水厂的取水点数量估计约为3万个(其中约95%为地下水源),但其中90%的取水点只贡献了21%的总水量。平均每天的取水量为1500万m3(每年5.4km3),其中2/3为地下水,1/3为地表水(见喀斯特,一种石灰岩中的可再生水资源)。
用于饮用水生产和供应的地表水资源中约80%为流动水体(河流、运河),其余20%为静水(包括来自大坝和水库的13%)。在基础设施方面,法国的饮用水生产和供应包括约3000座饮用水处理厂和处理站、数十万公里的输配水管道和超1000万立方米容量的清水池/地下水库/水塔(这些储水池的容积相当于一天的饮用水消耗量)。
建立生产饮用水的处理系统(或水厂)在于按照给定的顺序串连起若干水处理工艺(或处理单元),目的是为了生产出符合法规要求的让人满意的消毒过的水(见第4节),同时最大限度地减少处理副产物的生成。目前遇到的几个技术难题包括:水源水质极少是优质的、管网对输配水水质的影响、可用的工艺越来越多样化等。另一个需要克服的困难是消费者对饮用水口感要求的不断提高,以及(与其毫无担忧地饮用瓶装水形成鲜明相比的)对自来水水质的不信任。关注这一问题的媒体越来越多而使得这种不信任得以持续,但其来源有时是错误的。
自来水公司必须生产无味道的水,特别是无氯(消毒剂味)的水,尽管根据法规要求,饮用水在输配前和输配过程中需要进行消毒处理。尽可能减少氯味的办法只能是降低氯消毒剂或其衍生物的投加剂量。这要求在最后的消毒处理之前水质非常优良,因此需要进行有效的处理。
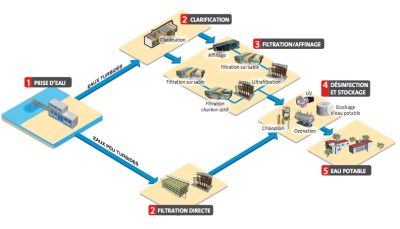
饮用水处理系统必须首先包括效果优异的消毒过程。如图2所示,在消毒之前包括三组可能的处理步骤:物理和化学预处理、澄清、深度处理。
预处理在地表水处理中非常常见。预处理可以在取水口或在处理厂进行,包括物理预处理(格栅筛选、沉砂、沉淀、除油、微细格栅)和化学预处理(用臭氧进行预臭氧:向待处理的水中注入包括氧气和臭氧(现场制备)的混合物实现水的消毒和污染物的氧化;再矿化包括通过往矿化度很低的淡水投加钙和碳酸盐使其矿化度得到提升(特别是为了防止管网腐蚀))。对地下水而言,预处理(当需要时)取决于水的特别性质。例如,氧化用于去除铁、锰和氨氮,而简单的曝气也可用于相同的处理目的。简单曝气还可用于脱气处理。
预处理后的一组处理步骤通常针对地表水进行。这一组步骤即为沉淀,包括混凝、絮凝、沉淀(或气浮)和过滤。对于某些低浊度水(湖水、冲积层地下水、喀斯特地下水),微絮凝单层(或双层)过滤(即混凝过程在滤池中完成),或者有机板式或管式膜材料过滤也许就足够了,其中膜过滤(详见“滤膜”)通过直接过滤物理截留水中颗粒物和微生物(微滤工艺使用的是能够通过直接过滤有效截留微米级颗粒的滤膜;超滤工艺使用的是能够通过直接过滤有效截留几十纳米量级颗粒的滤膜;纳滤工艺使用的是能够通过直接过滤有效截留几纳米量级溶解性颗粒和组分或者利用渗透现象分离溶解性组分的滤膜;反渗透工艺使用的是利用(反)渗透现象截留溶解性物质的滤膜,在高操作压力的驱动下可截留所有的溶解性物质,而在低操作压力的驱动下也可截留几乎全部溶解性物质)。
[图片来源:威立雅水技术照片库]
长期以来,深度处理阶段包括活性炭滤池过滤,以及(通常也采用的)前序臭氧氧化。活性炭可以是颗粒态或粉末态,由含碳物质材料(木材、煤、椰子等)在高温下煅烧而成,具有显著的吸附性能,特别表现在颗粒活性炭对有机微污染物(例如颗粒形态的杀虫剂)的吸附。深度处理也用于地下水的处理,特别是用于脱硝或者农药去除的过程。深度处理用于地表水处理时,是在传统水处理工艺之后加上一个或多个处理单元,使处理后的水质在某些化学物质(天然有机物、微污染物等)和微生物(病毒、寄生虫等)指标上得到提升。几乎所有的地表水深度处理工艺都采用这样的流程。近年来,传统的地表水处理工艺已经逐渐发生了改变,包括引入粉末活性炭(PAC)投加与液/固分离(超滤或高效沉降池)组合技术、纳滤或者低压反渗透技术。
4. 这些法规和控制是否符合任务要求?风险是什么?
饮用水准则一般由世界卫生组织制定,为全球饮用水水质提供指导。欧盟采用了世界卫生组织的饮用水准则,欧洲各国再以颁布法令和命令的方式在国家级别上对饮用水水质标准进行限定,并对部分指标进行了加强。它们由地方行政长官和大区卫生机构(ARS)实施,并最终由负责水生产和输配的人员(负责水生产和输配的PRPDE人员)和市长实施。当然,饮用水准则和法规的更深层次的目标是死亡率为零、发病率最低以及通过水质对公共健康做贡献。饮用水水质“标准”的建立有时是基于流行病学和毒理学方面的研究,但更多情况下是基于动物实验并外推到人类。所有水质标准的目的都是为了提供“好喝”的饮用水。
4.1. 欧盟推行的法规
当代版法国饮用水法规是基于1989年至1991年间颁布的法令(后者则是基于1980年7月通过的第一个欧洲指令),这些法令除了制定了微生物指标(“细菌检测”)外,还规定了许多化学指标的最高允许浓度。另外,这些法令还规定了与采样频率相关的水样分析内容,采样频率考虑了水的性质和管网服务的人口数量。
目前(2017年)正在生效的最新欧洲指令是于1998年12月通过的,该指令在2001年成为法国法律,然后在2003年引入至公共卫生法典中。该公共卫生法典会补充进一些定期发布的法令和行政命令。这些饮用水法规的范围涵盖所有用于(或经过处理后用于)饮用、烹饪、制作食物或其他家庭用途的水,不管这些水是如何输配的,包括“泉水”,但不包括“矿泉水”。
如引言所述,法规将饮用水定义为其中微生物、寄生虫或任何其它物质的数量或浓度水平不会对人类健康构成潜危害,并且水质指标低于由法令规定的最高允许浓度或参考值。为了达到这一目标,法规列出了一个消费者龙头水水质要求清单。这个清单包括大约60个指标,其中一半以“水质最高允许浓度”的形式进行规定,这些指标可能对消费者的健康产生急性或长期的影响;另一半以“水质参考值”的形式进行建议,这些物质在水中常见浓度的条件下对健康不构成直接影响,但可能表明水质发生变化和/或装置出现故障。
应当指出的是,这个清单中的指标,尽管其浓度经过分析且其浓度满足标准,就这些指标而言,还不足以彻底消除化学风险和病原微生物污染。因此,法规还在其它方面进行了规定,如水质保护和污染预防相关的技术条例、行政流程、水质控制流程、(及时)通知当局和消费者的条文、危机管理的条文等。
4.2. 除法规外,还需对输配水进行内部监控
内部监控必须由负责水的生产和分配的人员(PRPDE)确保完成,内容涵盖“水源、水处理、输配水”这一整套过程(译者注:即从源头到龙头)的性能和水质。在确定监控计划时需要包含的一个主要方面是原水和出厂的处理水(当然还有输配水)的水质(及其演变)。此外,还需包含:水厂和水系统层面设施的检查、纠正措施的实施、调查和研究、以及信息。这种内部监控是官方实验室在法规规定的条件下进行的监控的重要补充。
从微生物学的角度来看,由于法规仅对2种测试微生物(即大肠杆菌和肠球菌)进行“强制性”限定,即使满足这两项标准也并不能保证饮用水完全没有病原体。事实上,测试结果也无法证明水中完全和永远没有“微生物、寄生虫或任何对人类健康构成潜在危险的物质”,特别是存在饮水健康风险的人口的比例正在增加。此外,对一些微生物指标而言,其健康风险阈值(宣称为10-4)(译者注:即99.99%的去除率)在水质分析层面是无法实现的。例如,隐孢子虫就是这样,为了满足对它风险控制需求,需要采几十立方米的水样进行水质分析。
在化学品层面上,健康风险控制目前尚未将新出现的风险考虑进去,这些新风险来自于药物残留、化妆品、纳米颗粒等。水质控制的频率并不总是足够的,这一点对小的供水系统(供水规模不足5000名居民)而言尤为突出。对这些小系统,法规只要求有限次数的监控(每月不到一次)。而在法国这些小系统服务着1600万人口,其饮用水中有近12%在微生物指标方面不达标(对整个法国而言,饮用水中有4%在此方面不达标)。此外,对所有供水系统而言,确认了水质不达标对今后的供水水质仅是起预防性作用的,是对过去供水水质作出的反应。无论作出反应的速度有多快,“损害都已经造成”。因此,有必要根据出厂水水质以及预防性健康风险管理方法对现行健康风险控制进行补充。
因此,有必要要求在方法层面对法规的(水质)要求进行补充,以通过健康风险分析确保饮用水消费者能尽可能好地获得保护。健康风险分析必须包括危害的识别和表征以及暴露评估,如何通过技术和组织手段的实施进行风险管理(“多级屏障”理念),与各利益相关方进行沟通,同时分析可能的误解及寻求解决方案以进行补救。
5. 气候变化的影响
5.1. 关于可利用的水量

[图片来源:阿曼丁·里布雷奥 – 环境观察站,波伊图-查伦特斯]
目前有科学证据[4]表明气候变化已经在法国起作用,将对一些特别受此影响的地理区域的水资源产生重大和令人担忧的后果。例如,法国大西南部就是这样,据预测,到2050年,那里的天然河流的流量平均将减少20%至40%,地下水补给量将减少30%至55%。因此,可以想象到那时可利用水资源的紧缺情况,特别是在极端炎热和干旱期间。由此带来的后果是饮用水的使用和过量消耗方面的冲突将增加(见§ 2)。
5.2. 关于水源水质
在干旱或强降雨和持续降雨期间,地表水源水质会恶化,这一点也令人担忧。水温、营养物质和肥料的增加、以及相应的溶解氧的减少、可能的盐分增加,这些都将不可避免地影响水的微生物指标。水温变暖可能导致入侵物种(通常是嗜热的和条件性的)增加以及与内毒素和寄生虫相关的风险增加。

[图片来源:弗雷德里克·蒙蒂尼-地区环境观察中心]
此外,由于人为排放源水质和水量基本不变,因此污染物(在自然水体中)的稀释效果降低,加上原本已位于沉积物中的污染物可能发生再迁移,这些将导致有机和无机微污染物浓度的增加。地表水资源能否用于饮用水的生产和供应这个问题也将出现,特别是当这种水源是唯一可利用的水源时。哪些处理技术被认为能满足这类“原水”的处理需求的?这类原水有诸如寄生虫、藻毒素、天然有机物(有毒消毒副产品的前体物)、微污染物等水质问题。开发(有效但)能耗高的处理技术和更系统地使用化学试剂将劣质原水处理为“可饮用的水”是解决这些原水问题的方法,但以什么样的代价,会有什么(不利)影响?
气候变化对地下水水质的影响是什么?(农业使用的)肥料和用于植物保护的化学品(及其代谢产物)有相当大的比例会保留在土壤中,然后“逃离”进入地下水中。温度升高和极端水文事件会使更多肥料和化学品进入地下水。由于可利用的地表水资源量减少,越来越多的人口靠近海岸带居住,人均饮用水需求也在增加,(从地表水)转向地下水似乎是一个解决办法。就水质而言,地下水过度开发的主要风险将是污染物加速转移到更深的含水层,很多局部地区的盐水斜面可能上升。盐水斜面即沿海地区含水层中海水和淡水之间的边界。
5.3. 必要的适应计划
无论是对地表水还是地下水而言,应对气候变化的适应计划对于解决极有可能发生的天然水水质恶化问题至关重要。该计划将主要包括提高排放物的收集和净化水平,限制非点源污染和优化排放以补偿水资源数量的减少。该计划必须尽快执行,尽管会带来使饮用水生产(工艺)复杂化和自然环境受到危害的风险。
参考资料及说明
封面图片:公共领域。
[1] A WATER (2015). Water for a sustainable world, The United Nation Water Development Report 2015, 120 p.
[2] HERBET C., A. PICHON, B. JEUDI de GRISSAC, S. VAUZELLE, E. PARADES (2009). Lessons from the 2003 heat wave and the years 2007 and 2008 for taking climate change into account in estimating future drinking water needs. The challenges for the SAGE deep groundwater of Gironde. Symposium 193 SHF: “Drafts, droughts, rare heat waves and their impacts on water uses”, Lyon, 7-8 October 2009
[3] Extract from LEGUBE B. (2015). Drinking water production – Treatment systems and processes. Dunod Editeurs, Paris, 414 p. and digital supplement
[4] Examples:
BOURAOUI F., G. VACHAUD, L.Z.X. LI, H. LE TREUT (1999) Evaluation of the impact of climate changes on water storage and groundwater recharge at the watershed scale, Climate Dynamics, 15, p. 153-161.
DAYON G. (2015). Evolution of the continental hydrological cycle in France over the coming decades. Doctorate from the University of Toulouse Paul Sabatier. Thesis supervisors: J. Boé and E. Martin.
MEDDE (2016) EXPLORE 20170 Final Report http://www.gesteau.fr/document/bilan-du-projet-explore-2070-eau-et-changement-climatique
环境百科全书由环境和能源百科全书协会出版 (www.a3e.fr),该协会与格勒诺布尔阿尔卑斯大学和格勒诺布尔INP有合同关系,并由法国科学院赞助。
引用这篇文章: LEGUBE Bernard (2024年3月9日), 饮用水生产是一个重要的公共卫生议题, 环境百科全书,咨询于 2025年1月19日 [在线ISSN 2555-0950]网址: https://www.encyclopedie-environnement.org/zh/eau-zh/drinking-water-production-a-major-public-health-issue/.
环境百科全书中的文章是根据知识共享BY-NC-SA许可条款提供的,该许可授权复制的条件是:引用来源,不作商业使用,共享相同的初始条件,并且在每次重复使用或分发时复制知识共享BY-NC-SA许可声明。