磷与富营养化

磷是生命的基本元素,没有它,生命就不可能存在。磷对自然生态系统和农业生产至关重要。然而,人类活动(农业生产、废水排放、城市扩张、工业化)正在深刻地改变磷的循环。例如,过量的磷元素引起藻类爆发,然后在生物质的分解过程中消耗了其他物种生存所需的氧气,从而导致水生生态系统破坏:这就是所谓的富营养化。更重要的是,磷资源不可再生。根据全球范围内的磷消耗速度,磷矿预计将在一到两个世纪内耗尽。因此,控制环境中磷的流动,对于恢复退化的生态环境、保障粮食安全至关重要。本文包括了磷循环的关键机制、修复措施以及中长期磷资源的管理挑战。
1. 磷是如何循环的?
磷是生命物质(DNA、细胞膜、酶、骨骼、ATP)的必要组成部分,但它在自然环境中的含量很低,其含量低于陆地岩石质量的0.1%。它以磷酸钙、磷酸铁和磷酸铝等形式存在于火成岩和沉积岩中。在陆地表面,磷酸盐矿物通过岩石蚀变(矿物降解过程)在雨水等的作用下被溶解,植物吸收后通过生物合成过程形成有机物质,动物摄食植物,磷就在食物链中被逐级传递。最后微生物分解生物残体,磷被再次释放到环境中。
[图片来源:NASA。[CC BY-SA 3.0 (http://creativecommons.org/licenses/by-sa/3.0),Wikimedia Commons(法语版:http://www.astrosurf.com/luxorion/eco-terre-humus.htm)]
在小尺度(湖泊、河流、森林、牧场)下,磷在有机相(生物界)和无机相(生物分解后)之间演替。在更大的尺度范围内,磷通过水土侵蚀和淋溶进入生态系统:首先雨水将含磷溶质从土壤输送到地下水;然后含磷溶质通过河流运送到沿海地区。在磷的荣养下,沿海水域变得肥沃,通常盛产海洋浮游植物(图1)。在湖泊、河流、森林、牧场等小尺度下,磷在有机形态(生物界)和无机形态(生物分解后)之间转化。在更大的尺度范围内,磷通过水力侵蚀和淋溶进入生态系统,含磷溶质在雨水的作用下从土壤输送到地下水,再通过河流传输到沿海地区,促进了沿海水域浮游植物的大量生长(图1)。
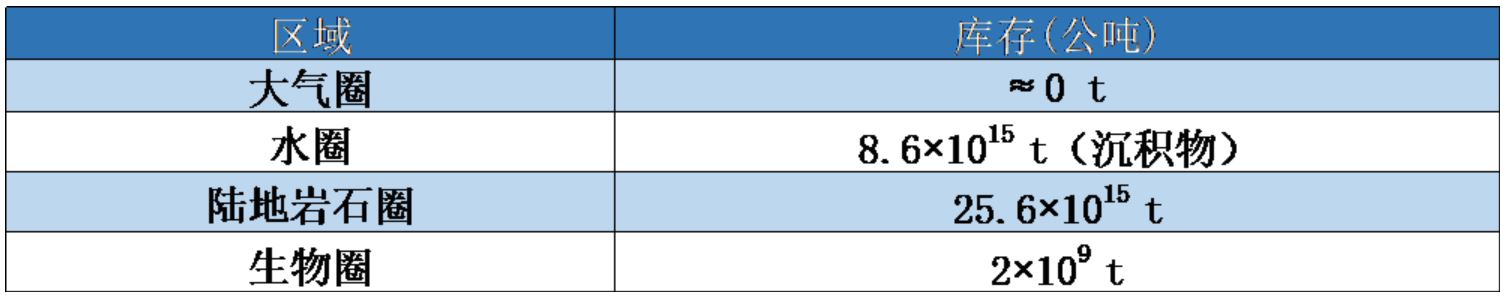
磷没有稳定的气态物质,与土壤和沉积物中的颗粒物有很强的亲和作用(表1)。通过水体搬运,陆地沉积物中的磷最终沉积至海底。因此,自然界的全球磷循环在陆地流失和海底积累之间并未达到完全平衡状态,从本质上讲,生物圈的磷循环是“开放的”。这显然与自然界的氮循环不同,因为氮在大气和地球其他圈层之间形成了真正的循环。通过开采磷矿、使用磷肥和含磷洗涤剂等产品,人类加剧了自然界磷循环的不平衡。
表1. 磷在地球各大圈层中的分布
2. 磷有哪些形态?
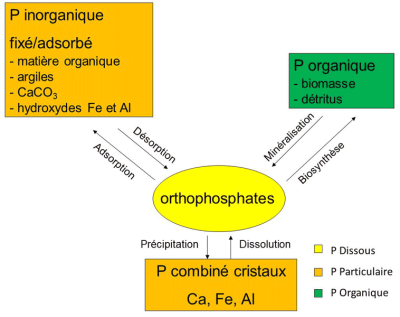
磷以溶解态或颗粒态的形式存在。溶解态磷包括无机形态的正磷酸盐离子(磷酸氢根离子HPO42-和磷酸二氢根离子H2PO4–)以及矿化过程(例如异养细菌降解有机残体中的蛋白和磷脂)中的有机形态。正磷酸盐离子(习惯上表示为PO)可以被植物吸收和同化,是植物唯一的生物可利用形式,因此对生态系统的功能发挥着至关重要的作用。正磷酸盐离子存在于土壤和底泥的孔隙水中以及环境水体中,被植物吸收后,通过生物合成过程转化为有机物。随后,在异养细菌矿化有机残体过程中,磷得以释放,再次回到土壤和水体环境。矿化过程中,异养细菌需利用水中的溶解氧来完成氧化和矿物质化过程。
图片文字翻译:
P inorganique 无机磷;fixé/adsorbé 固定/吸附;matière organique 有机质;Argiles 粘土;CaCO3 碳酸钙;hydroxydes Fe et Al 铁铝氢氧化物;Desorption 解吸;Adsorption 吸附。
P organique 有机磷;biomasse 生物质;détritus 残体;Mineralisation 矿化;Biosynthese 生物合成。
P combiné cristaux Ca, Fe, Al 磷的钙、铁、铝晶体化合物;precipitation 沉淀;Dissolution 溶解。
Orthophosphates 磷酸盐;P Dissous 溶解态磷;P Particulaire 颗粒态磷;P Organique 有机态磷。
在自然环境中,正磷酸盐的浓度非常低,譬如在高山湖泊或远洋海水中大约是10微克磷/升的量级。在受到人类活动高度干扰的环境中,它们可以达到几百微克磷/升。
颗粒态磷可以是有机或无机的。有机部分由生物合成,包括处于生命活动中和处于矿化过程中的有机态磷(图2),在颗粒态磷中占比很高,例如在农业区的河流底泥中占比高达50%。无机部分以两种形式存在:一是钙盐、铁盐或铝盐等最难溶的晶态磷,二是碳酸钙、氢氧化铁、氢氧化铝、粘土、有机质等颗粒物表面的固定态磷或吸附态磷。
通过吸附和解吸机制,固定态或吸附态磷可以被活化,与溶解态磷进行持续的动态交换。在水环境中,颗粒物的这种离子交换特性极大地影响了正磷酸盐的浓度。离子对颗粒物的吸附能力(总交换表面、颗粒大小等性质)非常敏感。因此,颗粒物对正磷酸盐离子浓度起着“缓冲”作用。
3. 有哪些磷来源于人类活动?
像所有生物一样,人类也需要磷。一个人每日通过食物摄入约1.5克磷。世界人口的增长极大地刺激了粮食需求。为了养活人类,农业在20世纪以来大幅发展,并逐渐集约化和工业化。为了保持农业系统的高生产率,必须不断向土壤补充被作物带走的磷元素。
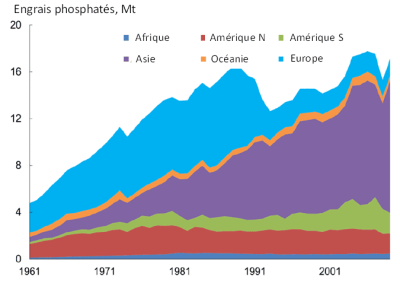
[图片来源:Van Dijk, K., Kabbe, C., Pellerin, S., Rechberger, H. & Oenema, O. (2013). 欧洲的磷使用情况。欧洲可持续磷会议。2013年3月6-7日。比利时布鲁塞尔。获取于phosphorusplatform.eu/component/jifile/download/MWFjMTAzNDViYzZhZhZjcxYzA4YTMyZTE1OTBhYjk2OWU=/2013-eu-sustainable-p-conference-p-use-in-eu-van-dijk-v3-pdf)]
图片文字翻译:
Afrique 非洲;Asie 亚洲;Amérique N 北美洲;Amérique S 南美洲;Océanie 大洋洲;Europe 欧洲。
瓜诺(鸟粪肥)的使用,以及20世纪起利用磷矿工业化生产磷肥,加速了全球磷资源的开采和使用。磷肥已在欧洲和北美洲推广,施肥量远超栽培植物所需。在各种理性消费的激励机制下,20世纪90年代欧洲在保持农业产量的同时,磷肥消耗量大幅下降,并从此保持稳定。另一方面,以中国为首的亚洲国家近几十年来在经济蓬勃发展,农业生产不断加强,目前成为最大的磷肥消费体(图3)。在不久的将来,非洲和南美洲国家的农业发展可能会进一步增加全球磷肥需求。
此外,1960年代以来,由于生产成本的降低,无机磷酸盐在食品、火柴、冶金、洗涤剂等日常工业产品中广泛使用。洗涤剂中的聚磷酸盐导致生活废水中的磷含量大大增加。自20世纪末以来,欧洲逐渐淘汰了聚磷酸盐。
近年来,磷肥的大量施用、耕地的侵蚀(面源)和生活污水排放量的增加(点源)都导致水环境中磷浓度的快速增加。在诸如过度施肥的农业土壤或富磷的河流底泥等环境介质中,大量的磷积累下来。了解地球表面磷储量的新分布,对于更好地管理这种有限的不可再生资源[1]至关重要。
4. 过量的磷和富营养化
几十年来,磷造成的环境污染(特别是水环境污染)备受关注。磷被认为是导致富营养化的主要原因。从词源学上讲,富营养化这个词的本义是“营养良好”;然而现在富营养化是指水体中营养物质过量(磷和氮),最终导致水体污染、生态失衡的现象[2]。富营养化表现为藻类生物量的增加,以及由其有机产物的异养矿化引起的水体脱氧。
富营养化波及河流、湖泊和沿海地区。此外,富营养化会破坏浮游植物的群落结构,其中增殖的甲藻和蓝藻等有害藻类可产生毒素。脱氧环境又使底泥中重金属和微污染物加速释放。这些效应关乎娱乐用水的安全性(有毒藻类)以及饮用水的生产和使用(阻塞抽滤器、在供水网中产生寄生动物、导致水中异味等),对经济生活造成一系列不利影响。

[图片来源:由Thesupermat(自创作品)][GFDL (http://www.gnu.org/copyleft/fdl.html) CC BY-SA 2.5-]
在生物合成过程中,藻类需要碳(C)、氮(N)和磷(P)。藻类的生长是由摄取营养元素的比例决定的(细胞中的Redfield摩尔比为C:N:P = 106:16:1)。在这个比率下,藻类就不存在生长限制。相对缺失的元素被称为藻类生长限制因子,例如氮或磷限制因子。对于藻类生长,磷通常会成为主要限制因子,尤其是在氮大幅度过量的淡水环境中。然而,在沿海水域中,氮(主要以硝酸盐形式存在;参见“环境中的硝酸盐”)则通常是导致富营养化的主要因素。例如,在布列塔尼(Brittany),农业源的硝酸盐导致了海滩绿藻的大量繁殖(图4)。
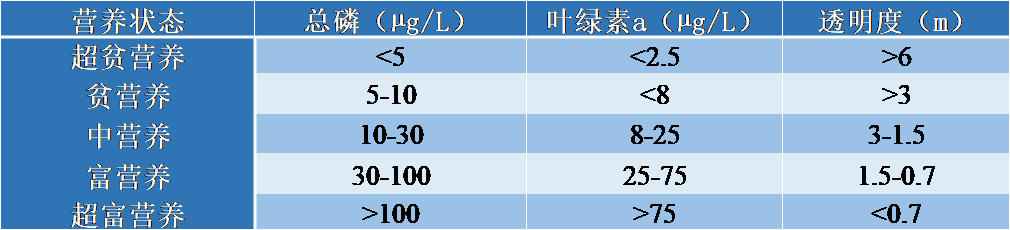
早在1960年代,世界各地就报道了诸多富营养化案例。这些研究首先在富营养化最严重的湖泊环境(包含湖泊和水库)中开展,如北美五大湖和欧洲大型高山湖泊(日内瓦湖、布尔热湖和安纳西湖)。在大量工作的基础上,研究者基于几个关键的水质指标(表2),将水体富营养化程度划分为超贫营养、贫营养、中营养、富营养和超富营养5个等级。
表2. 水体营养状态的分类阈值
这些指标的阈值可用于评定水体环境质量。贫营养是指由于磷缺乏导致的营养不良。相反,超富营养则是水体退化的最终阶段。中营养介于贫营养和富营养之间。在河流中,对富营养化问题的关注方兴未艾。人们往往认为河流是自净系统,能够自然消纳和排除水网中某个点的污染扰动。然而,随着微藻等浮游生物的增殖,富营养化成为大型河流中不容忽视的现实问题。此外,大量养分输入河流不可避免地会影响下游的峡湾、河口、泻湖和海岸带,而这些地方在全球范围内本身就饱受富营养化之苦。
5. 如何修复富营养化环境?
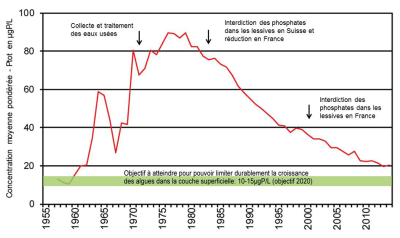
[图片来源:CIPEL图解(保护日内瓦湖水域国际委员会)www.cipel.org/themes/phosphore]
图片文字及翻译:
Collecte et traitement des eaux usées 污水收集及处理;
Interdiction des phosohates dans les lessives en Suisse et réduction en France 瑞士禁止在洗衣粉中使用磷酸盐,法国减少在洗衣粉中使用磷酸盐;
Interdiction des phosphates dans les lessives en France 法国禁止在洗衣粉中使用磷酸盐;
Objectif à atteindre pour pouvoir limiter durablement la croissance des alques dans la couche superficielle: 10-15µgP/L (objectif 2020) 为了可持续地限制表层藻类的生长,需要达到的目标:10-15微克磷/升(2020年目标)。
防治富营养化,首先是要减少点源磷的输入。在欧洲和北美的许多工业化国家,通过生活污水的收集、聚磷酸盐洗涤剂用量的削减和污水厂的针对性除磷,显著地减少了水环境中磷的输入。
从1980年代起,为恢复法国和瑞士边境的日内瓦湖水质,当地实施了卓有成效的点源磷的管控政策,使湖水中的磷浓度逐年降低(图5)。此外,包括河流及其支流排干的地理区域的流域面源污染也受到重点管控,以实现国际日内瓦湖保护委员会(CIPEL)所设定的富营养化防治目标。
农业土壤中磷的积累不容忽视。尽管采取了各种措施来限制土壤侵蚀(土壤侵蚀是指由于降水和径流导致土壤颗粒脱落和流失),并且减少了磷肥的施用,但农业面源污染问题仍未根除[3]。目前人们正在研究农业源磷的传输机制,考察其中的水文效应。对于磷的面源污染,从诊断问题、制定决策和采取行动,再到取得可观的修复效果,其过程长达数十年,可谓任重道远。
在减磷、控磷的同时,我们也许还应反思,水环境中磷的输入应该降低到何种程度为止?过度的减磷,是否反而对水生环境不利?减少磷输入的问题最近在欧洲引发了广泛争论。譬如日内瓦湖的渔民发现鱼类资源在连年减少,他们呼吁在湖中增加磷,以提高生态系统的生产力[4]。
虽然欧洲的富营养化正在减轻,但在城市急剧扩张、农业快速发展的其他新兴地区,由于未对环境质量给予足够重视,富营养化形势仍然严峻。
参考资料及说明
[1] Némery J. & Garnier J. (2016). The fate of phosphorus. Nature Geoscience, 9,343-344. (doi:10.1038/ngeo2702). Available at https://www.researchgate.net/publication/301272933_Biogeochemistry_The_fate_of_phosphorus
[2] Vollenweider, R.A. (1968). Scientific fundamentals of the eutrophication of lakes and flowing waters, with particular reference to nitrogen and phosphorus as factors of eutrophication. O.C.D.E. Paris, Technical Report, DA 5/SCI/68.27, 250 p
[3] Schoumans OF., Thistle WJ., Bechmann ME., Gascuel-Odoux C., Hofman G., Kronvang B., Rubæk GH., Ulén B., Dorioz JM. (2014) Mitigation options to reduce phosphorus losses from the agricultural sector and improve surface water quality: A review. Science of the Total Environment, 468-469, 1255-1266.
[4] 湖泊对鱼类而言太清澈了吗? 美国手语广告 n°84(2012年6月)
环境百科全书由环境和能源百科全书协会出版 (www.a3e.fr),该协会与格勒诺布尔阿尔卑斯大学和格勒诺布尔INP有合同关系,并由法国科学院赞助。
引用这篇文章: NEMERY Julien (2023年11月11日), 磷与富营养化, 环境百科全书,咨询于 2025年4月3日 [在线ISSN 2555-0950]网址: https://www.encyclopedie-environnement.org/zh/eau-zh/phosphorus-and-eutrophication/.
环境百科全书中的文章是根据知识共享BY-NC-SA许可条款提供的,该许可授权复制的条件是:引用来源,不作商业使用,共享相同的初始条件,并且在每次重复使用或分发时复制知识共享BY-NC-SA许可声明。