第一批复杂的生态系统

今天的生态系统在组成它们的有机体之间相互依存关系的复杂性方面令人叹为观止。贯穿食物网的能量和生物量循环将细菌、单细胞生物、植物/藻类和各种大小、秉性极为不同的动物关联在一起。这些生物系统的稳定性是以动态平衡为基础的,而动态平衡对环境和人为因子非常敏感。在30多亿年的时间里,海洋生态系统的组成一直以微生物(细菌、古菌)或单细胞真核生物为优势。尽管这些有机体在碳、氮的生物地球化学循环和提高地球氧含量方面发挥了关键作用,但它们从未形成如我们现今所知的自然界中的复杂食物网。5亿多年前,前寒武纪末出现的多细胞生物和宏体生物以及前寒武纪—古生代过渡期中动物界的出现彻底改变了海洋世界及其运作模式。
1.埃迪卡拉纪神秘的海洋生态系统

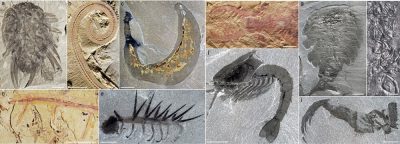
这些神秘的生物出现在加拿大(如米斯塔肯角)、澳大利亚(如埃迪卡拉)、纳米比亚和俄罗斯北部(如白海)等许多化石遗址点,它们在5.75亿年至5.42亿年前大量出现,并生活在不同深度的海底,该地质层段与埃迪卡拉纪末期相对应。由于沙质沉积物和火山灰的瞬间沉积,它们柔软、轻逸和灵活的躯体的印迹得以存留,而在当今自然界中没有与之对应的生物。
其中最为典型的叶状体生物[1]的特征是其波浪状的叶状体由柄牢牢固着在海底。然而,虽然这些固着生物有独一无二的构件和分形结构,然而的确达不到寒武纪的第一批动物解剖结构的复杂程度。它们显然没有口、消化系统和复杂的内部器官,因为它们具有与环境交换的巨大表面,被认为是通过直接吸收溶解的有机碳来获取食物(渗透营养)。
在埃迪卡拉纪,海底的水-沉积物界面也被海绵动物和许多身体扁平的生物所占据,后者有时会让人联想到某些软体动物(mollusc)和现今节肢动物(arthropod)的两侧对称(图1)。它们中的一些动物,如金伯拉虫、狄更逊水母和一种三叶虫纲动物(Yorgia)产生的一些痕迹化石表明,它们(这些扁平化的生物)能移动并以覆盖了整个海床的细菌膜(bacterial film)为食,正如扁盘动物(placozoaire)一样,它们可能沿着腹面进行体外消化。
当时的海洋生态系统以微生物垫和渗透性营养的多细胞生物(如叶状体生物)占优势地位,依靠滤食微型食物(microphage在术语中的意思为“小噬细胞”,在此显然不是该意——校订者注)(如海绵动物通过它们的过滤系统和鞭毛细胞)或利用外部接触消化为主要的方式。这些取食策略完全适应于前寒武纪末海洋中可获取的资源,即水-沉积物界面上随处可见的大量溶解的有机物和普遍存在的微生物垫。在前寒武纪—寒武纪过渡期,埃迪卡拉纪生物的灭绝并不是由像大多数重大生物危机那样的全球范围内动荡导致的,而是由于最早的掘穴动物(生物扰动作用)破坏了埃迪卡拉纪生物的生境。埃迪卡拉纪生物面对第一批捕食者完全没有防御能力,无法存活。
2. 第一个动物群落:现代生态系统的原型
顾名思义,寒武纪大爆发是指在化石记录中相对突然地出现了新的和解剖学上复杂的生物体,其中,我们可以肯定地识别出现今主要动物群的远祖(例如:节肢动物、蠕虫、软体动物、脊索动物,图2)。一些保存特别完好的沉积层(称为化石库),例如澄江(中国;约520 Ma)、伯吉斯页岩(加拿大;约505 Ma)、西里斯帕西特(格陵兰岛)和鸸鹋湾(澳大利亚)都向我们揭示了最早的海洋动物群落的存在。由于寒武纪早期的动物已经发展出运动和感觉能力(化石表明一些动物有头部神经系统),这些动物在环境中主动移动,并首次争相利用了多维生态位。这种动态标志着与埃迪卡拉纪非常重要的固着生活的海洋生物根本的区别,也是生态系统演化的一个不可逆转的转折点。

图中文字:EDIACARAN 埃迪卡拉纪,CAMBRIAN 寒武纪
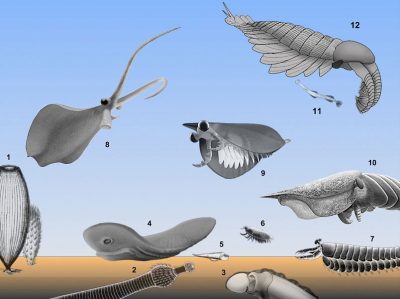
—刺胞动物,
—栉板动物,
—毛颚动物,
—原始软体动物和原始节肢动物(例如,等刺虫)
对这些化石及其现生后代的比较研究表明(图4),这些物种在原始食物链中相互作用[3] ,[4] ,[5] ,[6] ,[7] ,[8] ,[9] ,[10] ,[11] 。格陵兰岛下寒武纪的Timisiocaris物种就是这些新营养关系中的一个很好的例子。它是标志性的捕食者奇虾(Anomalocaris)的近亲,它的大型附肢上生有梳和过滤刚毛,使它能够捕获在水体中滤食生活的浮游动物。微化石证明了这种以真核藻类和细菌为食的这类浮游动物的存在。
然而,寒武纪海洋生物主要集中在水-沉积物界面,而海绵是固着动物群的主要组成部分。在所有保存完好的沉积层中,节肢动物是迄今为止最丰富和最多样化的附表底栖生物(图2)。它们的外部(分节,附肢)和内部(神经系统)结构图表明,其中一些与现今的甲壳动物和螯肢动物有亲缘关系。
另一些属于现已灭绝的类群。它们有着关节和多节外骨骼,这可能有利于其拥有许多功能和特化。寒武纪节肢动物拥有抓握足和咀嚼附肢使其能够捕获猎物和减少产生食物颗粒。滤食巨型食物的取食方式(macrophagia)出现在寒武纪时期的许多食肉动物或以尸体为食的动物(食腐动物)中。例如,伯吉斯页岩(Burgess Shale)的节肢动物西德尼虫(Sidneyia)捕获、粉碎并食用小型三叶虫,其附肢和胃内容物如图所示(图2)。
其他新特征的出现也促成了食物链发生重大变化。消化腺可提高酶降解食物的效率,从而促进滤食巨型食物的取食方式出现在许多寒武纪的节肢动物中。视觉也彻底改变了早寒武纪海洋生物之间的相互作用。例如,由数千个小眼组成的大型复眼使超级捕食者奇虾(Anomalocaris)能够发现并追踪它的猎物。毫无疑问,在寒武纪节肢动物中普遍存在的视觉已经显著改变了猎物-捕食者的关系,并在生态系统中引入了新的选择压力,导致了多种适应性反应。
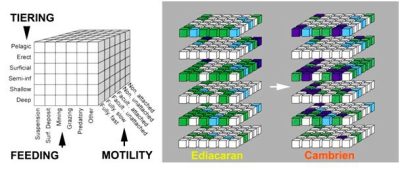
图中文字: TIERING分层;Pelagic栖居上层的,浮游的;Erect (底栖)挺水的;Surficial(底栖)表栖的;Semi-inf (底栖)半内栖的;Shallow (底栖)浅内栖的;Deep (底栖)深内栖的;FEEDING 取食;Suspension 滤食;Surf Deposit沉积物表面取食;Mining 掘食; Grazing 牧食;Predatory 捕食;Other其他;MOTILITY活动水平;Non. Attached 非游动的,固着的;Non. Unattached 非游动的,未固着的;Facult. Attached 兼性固着的;Facult. Unattached 兼性非固着的;Fully Slow自由,缓慢运动;Fully Fast自由,快速运动;Ediacaran 埃迪卡拉纪;Cambrien寒武纪
参考资料与注释
封面图片。埃迪卡拉海洋的生命 [图片来源:© Ryan Somma (CC BY-SA 2.0),,经由维基共享资源]
[1] Narbonne G.M., Laflamme M., Greentre C. Trusler P. (2009) Reconstructing a lost world: Ediacaran rangeomorphs from Spaniard’s Bay, Newfoundland. Journal of Paleontology 83, 503-528.
[2] Fedonkin M.A., Gehling J.G., Grey K., Narbonne G. Vickers-Rich P. (2007) The Rise of Animals. The Johns Hopkins University Press, Baltimore. 325 pp.
[3] Briggs D.E.G. (2015) The Cambrian Explosion. Current Biology 25, R864-R868
[4] Caron J.-B., Scheltema A., Schander C. & Rudkin D. (2006) A soft-bodied mollusc with radula from the Middle Cambrian Burgess Shale. Nature 442, 159-163.
[5] Caron J.-B., Conway Morris S. & Cameron C.B. (2013) Tubicolous enteropneusts from the Cambrian period. Nature 495, 503-506.
[6] Daley et al (2009) The Burgess Shale anomalocarididid Hurdia and its significance for early euarthropod evolution. Science 323, 1597-1600.
[7] Hou X.-G., Aldridge R.J., Bergström J., Siveter David J., Siveter Derek J. & Feng X.-H. (2004) The Cambrian Fossils of Chengjiang, China. Blackwell Publishing. 233 pp.
[8] Smith M.R. & Caron J.-B. (2010) Primitive soft-bodied cephalopods from the Cambrian. Nature 465, 469-472.
[9] Vannier J., Steiner M., Renvoisé E., Hu S.-X. Casanova J.-P. (2007) Early Cambrian origin of modern food webs: evidence from predator arrow worms. Proceedings of the Royal Society London B 274, 627-633.
[10] Vannier J., Garcia-Bellido D.C., Hu S.-X. Chen A.L. (2009) Arthropod visual predators in the early pelagic ecosystems: evidence from the Burgess Shale and Chengjiang biota. Proceedings of the Royal Society London B 276, 2567-2574.
[11] Vinther J., Stein M., Longrich N.R. & Harper D.A.T. (2014) A suspension-feeding anomalocarid from the Early Cambrian. Nature 507, 496-500.
[12] Bambach R.K, Bush A.M. & Erwin D.H. (2007) Autecology and the filling of ecospace: key metazoan radiations. Palaeontology 50, 1-22.
[13] Erwin D.H. & Valentine J.W. (2013) The Cambrian Explosion: the construction of animal biodiversity. Roberts & Company Publishers. 406 pp.
环境百科全书由环境和能源百科全书协会出版 (www.a3e.fr),该协会与格勒诺布尔阿尔卑斯大学和格勒诺布尔INP有合同关系,并由法国科学院赞助。
引用这篇文章: VANNIER Jean (2024年3月13日), 第一批复杂的生态系统, 环境百科全书,咨询于 2025年4月2日 [在线ISSN 2555-0950]网址: https://www.encyclopedie-environnement.org/zh/vivant-zh/first-complex-ecosystems/.
环境百科全书中的文章是根据知识共享BY-NC-SA许可条款提供的,该许可授权复制的条件是:引用来源,不作商业使用,共享相同的初始条件,并且在每次重复使用或分发时复制知识共享BY-NC-SA许可声明。









