放射性与核反应
要使原子核稳定,核内的中子和质子需要保持一定的比例。否则,原子核将经历一系列衰变,通过失去多余的粒子以达到一个稳定的状态。在这个过程中,原子核将产生辐射,如果辐射剂量高,将会对未加保护的活体组织造成损害。每个放射性核具有独特的辐射性质和能量特征以及特定的放射性活度。
1. 原子核:从稳定到放射性
固体、液体、气体[1](图1)是由原子直接组成或是由原子先相互结合形成晶体、分子再组成的。原子由原子核和很小的带负电的核外电子组成,其中原子的质量大部分集中在原子核上。实际上,原子内部十分空旷:如果把原子放大到一个教堂中殿那么大,原子核相当于一只在唱诗班中间巨大的嗡嗡作响的雄蜂。
原子核也是由许多重质量的粒子组成的,这些粒子叫做核子,其质量是电子的1800多倍。核子还可以分为带正电的质子和不带电的中子。核中质子的数量决定了这个原子是哪种化学元素(例如:1个质子=氢,2个质子=氦,6个质子=碳)。这个质子数就是原子序数,记为Z;而核子的总数量,也就是原子质量,记为A。Z相同而A不同的原子叫做同一种元素的同位素,它们的化学性质(与质子数有关)几乎完全一样,但它们的核性质可能天差地别。同位素通常用在其元素符号上标注其原子质量的方式来标记,如:1H,4He,12C,16O,235U。
电子或近或远地分布在原子核外的各层上。外层的电子在化学反应中起着重要作用。在分子中,原子不同程度地共享着这些外围电子。如果原子的质子数和电子数相等,它就是电中性。如果这两数不相同,这个原子就被电离了,但它仍保留着原有的化学性质。如果电子数大于质子数,它就是负离子,也叫做阴离子(例如:Cl-,O-);若电子数小于质子数,它就是正离子,也叫做阳离子(例如:H+,Ca++)。
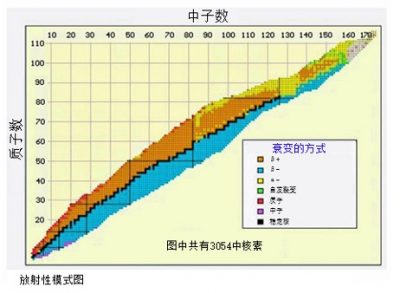
在原子核中,中子确保了核子能够结合在一起,不至于因带相同电荷的质子互相排斥而解体。原子核中,核子越多,中子数就要超过质子数越多,这样核子们才能稳定地结合。因此,在对比原子核中质子数和中子数的图表上,自然界中原子的原子核落在所谓的“稳定谷”中(图2)。
如果原子核的中子太多或太少,它就会不稳定,会或快或慢地向稳定谷移动,并按照下一章我们所讲的一种机制进行分解。因为分解的同时会发射出能量和辐射,所以也叫做放射性。
天然放射性常常被当成与人工放射性相对的概念,但这只是语言的滥用。放射性就是放射性,不过是可以由地球上自然存在的原子核产生(人体内就天然存在着钾40K和碳14C),和由粒子加速器或核反应堆中人工制造的原子核产生的区别罢了。天然放射性元素可以直接从恒星和超新星的核合成中产生,例如钾40K和铀238U,也可以从以前存在的原子核和宇宙辐射粒子的相互作用中产生,例如碳14C。此外,它们也可以从另一种放射性原子核的衰变中产生,例如镭就是铀衰变的产物。
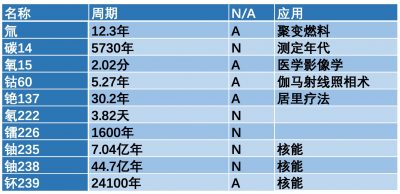
每种放射性同位素(或称为放射性核素)的特征是衰变速率。这个速率以周期来量化,有时也叫半衰期,记为,即给定数量的原子核在经过多长时间后正好有一半衰减了。衰变的周期是一种统计特征,对于单个的放射性原子核,我们无法预测它会在什么时候发生衰变,但如果有十亿个这样的原子,我们能极其精确地知道在什么时候将剩下五亿个原子。不同原子核的这种特征周期是几毫秒到几十亿年不等(参见表1)。
在媒体口中,衰变周期一般被叫做“寿命”,相关评论还会暗示放射性原子的寿命越长就越危险。实际上恰恰相反,衰变周期长,是因为衰变速率低,也就意味着放射性活度低。
2. 辐射、活度、剂量
放射性原子具有通过发射辐射物回到稳定谷的趋势。它们的辐射有以下几种不同方式(见图3)。
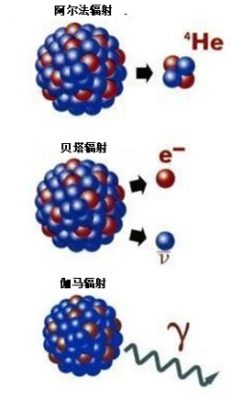
2.1. 阿尔法辐射(α)
如果原子核因为有太多核子而不稳定(通常是发生在重元素上),这个放射性原子将分解并发射出一个由两个质子和两个中子组成的粒子,称为α粒子,也就是一个氦核。在这种辐射中,衰变后的剩余核与衰变前的原子核相比,原子序数减少了2,质量数减少了4,例如:钚239Pu衰变为铀235U,铀235U衰变为钍231Th。
2.2. 贝塔-辐射(β-)
如果放射性原子因为有太多中子而不稳定,它会把自己的一个中子转变为质子,同时发射出一个电子以保持电中性,以及一个称为反中微子的微小粒子(记为)。这样一来,原子质量数不变,原子序数增加1,称之为β-辐射。例如,碘135I衰变为氙135Xe,镎239Np衰变为钚239Pu。
2.3. 贝塔+辐射(β+)
在少数情形下,原子核可能会缺少中子。此时核中的一个质子将会变为中子同时发射出一个正电子(带正电的电子)和一个中微子。这样,原子质量数不变的同时,原子序数减少1,称之为β+辐射。
2.4. 伽马辐射(γ)
即使核子的数量都合适,原子核也能被激发,不得不释放多余的能量,发射波长很短的电磁辐射——一个光子γ(参见《天空的颜色》)。β衰变通常伴随γ辐射。还有一些更罕见的放射性形式,例如,发射中子(见文章《核能利用》的“缓发中子”部分)。
衰变之后,产生的原子可能仍不稳定且能继续衰变。在达到稳定状态之前,连续的衰变将会像“链式”环环相扣。例如铀238U经历16次衰变后,会最终变成稳定的铅208Pb。
2.5. 放射性的单位
放射性原子的活度指的是其衰变的速率,单位是贝克勒尔(简称贝克,Bq):1Bq=每秒发生1次衰变。贝克是一个非常小的单位,前一章提过的人体的天然放射性大约是8000Bq,绝没有到为了不辐射你的邻居而穿铅外套的程度!这也是为什么放射源活度的单位常常是千兆(十亿)贝克(GBq)甚至太(万亿)贝克(TBq)。活度只度量放射的节奏,而不度量放射的效果。试想你被乒乓球和法式滚球(通常为钢球)以相同的节奏轰击,效果自然不同!因此我们下面将提出剂量的概念。
当辐射照射到物质时,其部分或全部的能量将逐渐失去并被物质吸收。一定质量的物质所吸收能量的量称为剂量。剂量的单位是戈瑞(Gy):1戈瑞=1焦耳每千克。与贝克不同,戈瑞是非常巨大的单位,所以通常使用的微(百万分之一)戈瑞和毫(千分之一)戈瑞这样的分数单位。
在生物组织内(见下文),辐射造成的伤害不仅取决于剂量,也取决于辐射的性质,以及被辐射组织的性质。如果说剂量是一个物理量,那么当量剂量就是一个生物量。当量剂量通过把戈瑞数乘上一个合适的系数来体现这些额外因素的影响。当量剂量的单位是希沃特(简称希,Sv),常用的是毫或微沃特(mSv,μSv)。
剂量率度量的是单位时间吸收的剂量(例如:μSv/小时,mSv/年等)。
3. 辐射对生物体的影响
3.1. 如何保护你自己
辐射很可怕,因为我们的感官感觉不到它——它无形无味无声,更因为高剂量的辐射能引发癌症。
另一方面,虽然感官无法感觉到,要检测到辐射却十分容易,即便只是很小的剂量:做PET检查时[2],我们可以记录下每一个单次的衰变!相比之下,化学毒物的分子数必须要达到几百亿亿,否则没有任何仪器能将其检测出来!
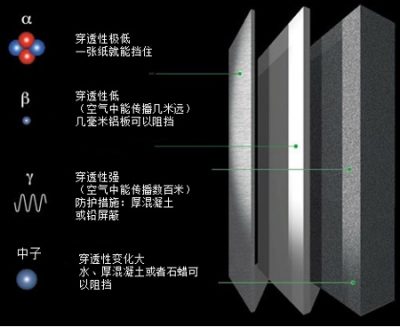
此外,只要确定了辐射源,要保护好自己还是很容易的:首先,不要靠近辐射源;如果你不得不接近它,也不要久留;最后,如果你的工作需要你长期待在辐射源附近,你可以设置一个屏蔽层来防止辐射,如图4所示。
- 一张卷烟纸、一小层我们表皮上的死细胞就足以阻挡α射线。
- 几毫米厚的铝板就可以阻挡β射线。
- 1.5米厚的混凝土,或5米厚的水可以阻挡伽马射线和中子。

正因此,一些实验性的核反应堆建在了水池底部(见图5)。操作员待在水池上方,即使让反应堆满载,由于水阻挡了所有反应堆的辐射,而厚厚的建筑物又包裹着反应堆,保护了操作员免受宇宙射线的辐射,所以他们受到的辐射比常人还少。
3.2. 辐射的生物效应
因为放射性物质的辐射具有较高的能量,在进入物质的时候,它可以电离原子(吸走电子)。这也是为什么它们被归类为“电离辐射”,与可见光和波长较长的电磁辐射相对应(参见《天空的颜色》)。电离辐射引发的电离能够破坏分子并引发化学反应。宇宙射线,即太阳或是更远的天体所发射的高能粒子,以及医学影像中使用的X射线,都是电离辐射。
我们体内的大部分分子都在不断地循环再生,电离辐射对它们的影响是可逆的。但DNA不同,我们细胞中的DNA负责携带遗传信息,所以保护好DNA是至关重要的。DNA是一个由几十亿原子组成的双链形成的双螺旋:电离辐射可能会伤害其中一条,甚至两条链。
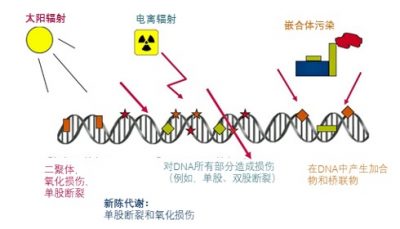
如图6所示,电离辐射不是DNA面临的唯一问题。长远来讲,最大的问题是我们的新陈代谢:呼吸、消化,都会让我们产生大量的自由基,尤其是活性氧,仗着自己强大的化学活性就肆无忌惮地攻击我们的DNA。我们体内每个细胞里的DNA每天要被新陈代谢的产物攻击10000到30000次!
也就是说,我们身体的修复机制极其高效迅速。相比之下,天然放射性每年也就攻击我们每个细胞几次而已。
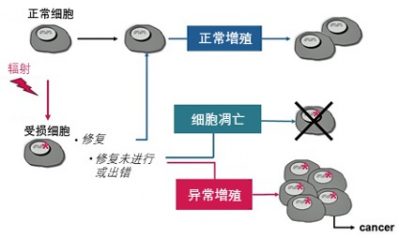
如果我们的DNA被伤害会怎么样呢?可以参见图7(以及《太阳光中的紫外线对细胞的影响》)。大多数情形下,DNA都能被修复,然后细胞继续过完正常的一生。如果细胞检测到修复没有进行或是出错,它一般会自杀,这是一种称为“细胞凋亡”的机制,这种机制在胚胎学中起着重要作用[3]。如果被破坏的细胞很少,就不会有影响。但在一些罕见的情形下,异常细胞会开始增殖,这就是癌症的开始(贪婪的癌细胞只有得到足够的营养,并且摆脱了所处组织和身体的诸多控制机制时才会引发癌症)。
可以看出,单次辐照确实能引发癌症,但概率极其低。
3.3. 国际辐射防护规则
联合国原子辐射效应科学委员会(UNSCEAR)成立于1955年,周期性地对其职能领域内的所有国际科学数据进行总结[4]。从一开始,UNSCEAR就在进行“同层人”的流行病学研究。“同层人”指遭受了重大辐射和污染的群体,包括广岛长崎的幸存者、前苏联玛雅克(Mayak)设施的工人、特查(Tetcha)河两岸的居民、塞米巴拉金斯克(Semipalatinsk)空中原子弹爆炸现场附近的居民、美国的放射学研究人员、飞机上的机组成员(飞行时会暴露在宇宙射线中)等等。正是基于这些研究,人们制定了辐射防护的规则,以保护公众和工作人员免受辐射危害。总的来说,辐射的影响中的变量基本上是辐射剂量,也与体现剂量强度的“剂量率”有关。
3.4. 高剂量
- 如果一个人受到了高剂量(1Sv以上)的辐射,会有很多细胞死亡,其影响将是肉眼可见的:皮肤、血液、肠道等都会出现病变。剂量越高,病变就越严重。这种现象被称为“急性辐射综合征”,是一种确定性效应。
- 此外,如果一群人被高剂量辐射照射,他们会比没有被辐射照射的人更容易得癌症。得癌症的概率与辐射剂量有关,但癌症的严重性与之无关。这种因人而异的效应称为随机性效应。
3.5. 低剂量
高剂量带来的危害显而易见,但我们仍不清楚低剂量的危害。一方面,除了电离辐射,还有许多其他的致癌因素;另一方面,在天然放射性的背景噪声中,我们辨别不出低剂量的辐射。而且,我们很难找出个体差异只存在于辐射剂量上的同层人:比如,对于一个不吸烟的人,需要非常大的辐射剂量才能使其癌症风险与吸烟者相同。虽然如此,我们可以这样认为:
- 在100 mSv(1Sv)以下的剂量中,没有观察到癌症病例的增加,但如果受辐射且被观察的人群数量更大的话,也许能观察到一些。
- 这也是为什么国际放射防护委员会(ICRP)为了确定剂量限制,将高剂量下观察到的影响线性关系外推到低剂量范围。根据预防原则,这种保守的“无阈值的线性关系”的假设被用来制定辐射防护规则。这么做可能会忽略了可能存在的阈值效应,从而过高地估计平均发病率:我们之前提到过的修复机制在低剂量时更高效,在高剂量时却达到“饱和”。此外,高剂量辐射对一群人的影响可以极大地掩盖他们的个体差异,出现的癌症病例数呈现出这种罕见事件固有的随机波动:仅有一百多例,接近平均值。
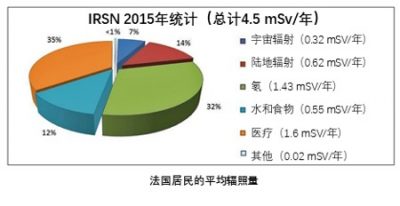
在法国,核活动不得使公众受到超过1mSV/年的辐射,相关工作人员不能超过20mSv/年。
法国的天然放射性产生的剂量在2.4到10mSv/年之间,在世界上的一些有特殊放射性土壤的地区,天然辐射的剂量能超过100mSv/年。法国人均在医用和牙科x光片上受到1mSv/年的辐射。图8展示了巴黎盆地居民受到的平均辐照量。
3.6. 污染
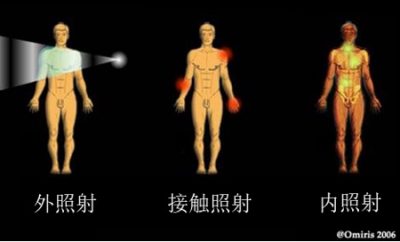
前文讨论的是辐射,或者说是外照射。图9展示了其他可能的受辐射模式。如果吸收了放射性元素,就是内照射或者污染了。这时离开辐射源或者设置屏障已经晚了。即使是几厘米厚的空气或是表皮就能阻挡的阿尔法射线现在也能把它所有的能量沉积在组织和器官中。
内照射有三种发生形式:吸入放射性物质或粉尘;摄入含有放射性的食物或将有放射性的物质放入口中;放射性物质造成外伤或现有伤口被污染。人体内被污染是很难[5]被净化的。不过有时其后果可以减轻,例如被氚污染后,可以通过大量喝水降低伤害。

作人员通过穿特殊的工作服,佩戴手套和面罩(使用后都会成为放射性垃圾)来保护自己免受污染。存有放射性物质的房间要密封,房间里换气时要过滤掉放射性粉尘。高放射性物体通过远程操纵器隔着屏障来操作,有时也用机器人。
例如,钚燃料的制造步骤与浓缩铀燃料的相同(放射性很微弱,参见《核能利用》),但操作必须在被称作手套箱的密闭罩中进行(见图10)。
本章的最后,我们必须提及特高剂量的辐射在医学上的应用:放射治疗和近距离放射治疗。辐射治愈的癌症远比它引起的多。
参考资料及说明
封面图片:皮埃尔·居里(Pierre Curie)和玛丽·居里(Marie Curie),他们与亨利·贝克勒尔(Henri Becquerel)因发现放射性共同获得1903年诺贝尔物理学奖。
[1] 第四种物态:等离子体,它构成了99%的宇宙。等离子体中没有原子,原子核们在电子的海洋中遨游。
[2] 正电子发射断层成像术,可以显示诸如被特定活动激活的大脑区域。
[3] 例如,在手的形成过程中,并不是手指长出来,而是手指间的细胞自杀了。
[4] 可以在IRSN报告2006年第74期上找到一篇UNSCEAR报告的法文摘要。
[5] 这是可能的。例如,使用螯合剂(钳子形状的特殊分子)洗肺来去除吸入的钚粒子,但过程十分繁琐。
环境百科全书由环境和能源百科全书协会出版 (www.a3e.fr),该协会与格勒诺布尔阿尔卑斯大学和格勒诺布尔INP有合同关系,并由法国科学院赞助。
引用这篇文章: BARRÉ Bertrand (2024年4月23日), 放射性与核反应, 环境百科全书,咨询于 2025年4月8日 [在线ISSN 2555-0950]网址: https://www.encyclopedie-environnement.org/zh/physique-zh/radioactivity-and-nuclear-reactions/.
环境百科全书中的文章是根据知识共享BY-NC-SA许可条款提供的,该许可授权复制的条件是:引用来源,不作商业使用,共享相同的初始条件,并且在每次重复使用或分发时复制知识共享BY-NC-SA许可声明。