人类微生物群:我们健康的盟友

人体是整个微生物群落(细菌、古细菌、酵母菌和病毒)的家园。如今,它们被归为“微生物群”,参与许多生物学功能。因此,我们与它们发展出了一种真正的互利共生关系。在我们的皮肤(皮肤微生物群)、口腔(口腔微生物群)、生殖器(阴道微生物群)和肠道(肠道微生物群)中都有微生物存在。我们很容易认为细菌存在于与外界接触的区域(如皮肤、口腔),但事实上,我们的消化道也包含大量细菌,这有点令人费解,尤其是在像结肠这样很深的区域,每克肠道内容物所包含的细菌超过1011个。总的来说,我们的肠道微生物群包含100,000亿个微生物,它们对维持我们身体的正常运作和健康起着重要作用。因此,人类是一个由人体细胞和微生物组成的复杂生态系统。如果微生物群一直被忽略,会怎样呢?
1. 人类和他们的微生物群
人类不止拥有一种微生物群,而是多种微生物群。事实上,相同的微生物并不存在于身体的所有部位,它们的增殖完全取决于其寄居的地方。营养来源、湿度条件、有氧与否,这些因素都影响了在特定区域增殖的微生物的性质。因此,人体内有皮肤微生物群、口咽微生物群、阴道微生物群或肠道微生物群。每个微生物群所占据的生态位可能非常小,例如特定的皮肤区域、腋下或背部皮肤。
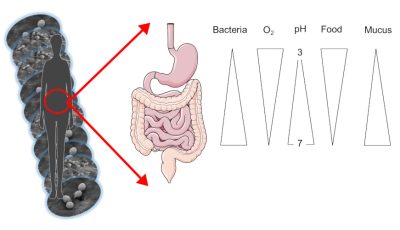
皮肤微生物以皮脂 皮肤上的小腺体所产生的物质,由脂质组成,充当皮肤的保护层和死亡的角质细胞 主要存在于皮肤上的细胞为食。它在例如腋下或皮肤皱襞这类潮湿的区域和背部皮肤这类干燥但油性的区域进化。同样,我们的肠道微生物群也根据其在消化道(顶部或底部)的位置而变化。事实上,胃是酸性并富含氧气的,而肠道末端的结肠是完全缺氧且无酸的。
微生物的数量,尤其是细菌的数量,也因占据的区域而异:从胃里1000个细菌到结肠中的1011个,再到每平方厘米皮肤里的100万个!总的来说,整个消化道包含了1,000,000亿个细菌,而在成年人体内仅肠道微生物群就重达1.5千克(图1)。
1.1. 细菌,我们微生物群的主要微生物
微生物群是指由酵母、古菌、真菌、病毒和细菌组成的复杂微生物群落。细菌是最丰富的,这就是为什么微生物群有时等同于细菌群。细菌是目前为止最广为人知、被描述最多的,尤其是在消化道远端部分(结肠)的细菌。例如,来自消化道的酵母菌占肠道菌群的比例不到0.5%,而细菌则占90%以上。所有这些微生物都生活在群落中,并与细胞相互作用,在这些相对丰度不断进化的种群之间维持一个动态平衡。有些种群在漱口或抗生素治疗后会发生变化,其他种群会借机占据空间并建立一个生态位。形成微生物群的种群动态源于与环境的相互作用。微生物群可以由组成它们的微生物来描述:在某一特定时间建立一个当前物种及其数量的“地图”。
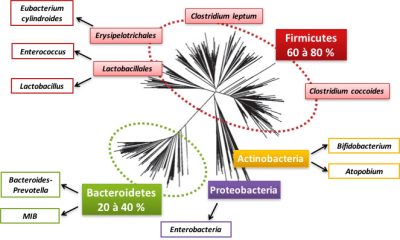
Firmicutes 厚壁菌门60~80%
Erysipelotrichales 丹毒丝菌目:Eubacterium cylindroides 圆柱状真杆菌;
Lactobacillales 乳杆菌目:Enterococcus 肠球菌,Lactobacillus 乳酸菌;
Clostridium leptum 柔嫩梭菌,Clostridium coccoides 拟球梭菌,
Actinobacteria 放线菌门:Bifidobacterium 双歧杆菌,Atopobium 奇异菌属;
Proteobacteria 变形菌门:Enterobacteria 肠道菌;
Bacteroidetes 拟杆菌门20~40%:Bacteroides prevotella 普雷沃氏菌属,MIB
我们可以通过粪便(粪便样本)获取大部分肠道微生物群;虽然它们很容易获得,但肠道微生物群长期以来一直不为人知。原因很简单:微生物,尤其是肠道中数量众多的细菌,很难生长,因为它们对氧气非常敏感,氧气会杀死60%以上的微生物。这就是为什么他们喜欢呆在消化道的下部(即结肠),因为在这个区域,没有氧气!这些细菌也有对食物的偏好和营养需求,但目前我们仍对此知之甚少。如果他们无法通过实验室培养大量获得,科学家们可以解密他们的DNA。针对微生物群的主要高通量DNA测序研究于1995年至2005年间启动[1]。这些研究计划使我们能够创建体表和体内的微生物群及其所携带基因的精细图谱(图2)。不同的分子生物学和测序方法已经被用于开发代表生态系统中优势和亚优势细菌的所有基因组的所有序列的“元基因组”(参见表征生物多样性的DNA条形码)。因此,肠道元基因组使我们能够根据个体的健康状况、遗传基因、饮食和生活条件,创建一个代表个体的细菌基因目录。
除了元基因组方法外,测序还可以通过靶向编码小核糖体亚基(16S)的基因来更加精准地描述微生物群中存在的细菌种类。这使得表征物种并评估它们的相对数量成为可能。
1.2. 21世纪:征服人类微生物群
让我们来详细介绍一下居住在我们身上的细菌世界。肠道微生物群包含800至1000种细菌,其中大部分属于两个细菌群(或门):厚壁菌门和拟杆菌门(见图2)。也存在变形菌门、放线菌门、疣微菌门、梭杆菌门,但数量较少(图2)。在这些细菌门中,有细菌科,如厚壁菌门的乳酸菌科。在这些细菌科中,我们可以区分出细菌种,例如拟球梭菌。绝大部分细菌种是个体特有的。古菌[2]通常存在于肠道微生物群中,尤其是那些参与了甲烷产生的微生物群。
一个人的微生物群被认为与其指纹一样独特!然而,一些物种被认为是“创始人”,例如普拉梭菌或嗜黏蛋白阿克曼菌,它们存在于大多数个体中,它们的缺失可能会导致一些病症。
人类细胞的DNA包含了组成25000个基因的近30亿个碱基,但细菌只有构成2000到3000个基因的约100万个碱基。如果我们将人类细胞与细菌进行比较,那么细菌的遗传能力要低10倍。然而,如果我们考虑到组成我们肠道微生物群的500到1000个物种,细菌可以表达的基因是其宿主的100到150倍。
针对微生物群的主要DNA测序计划于1995年到2005年间启动(见[1]),这使得绘制其基因的详细图谱成为可能。我们已经创建了目录对人类肠道微生物群不同细菌种类中的300万至1000万个基因进行分组。这些基因允许细菌执行特定的功能(消化营养物质,产生维生素……),并且是人体正常运作所必需的。因此,微生物所携带的遗传物质越多样,微生物群的代谢潜力就越大。在不同个体之间,构成肠道微生物群的物种多种多样,但细菌执行的功能可能是共通的,也可能是多余的。
这些微生物群中的细菌基因目录使人们能够根据年龄、生活条件和健康状况对个体或群体进行比较。肠道微生物组基因的多样性甚至可能是某些疾病易感性的标志:肠道中所携带的细菌基因种类越多,对您的健康越有益。
1.3. 人类“口腔”、“阴道”和“肺部”微生物群
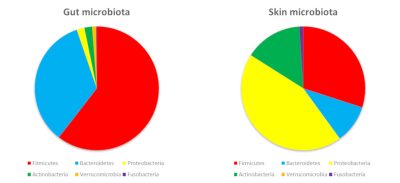
Gut microbiota 肠道微生物群,Skin microbiota 皮肤微生物群,Firmicutes 厚壁菌门,Bacteroidetes 拟杆菌门,Proteobacteria 变形菌门,Actinobacteria 放线菌门,Verrucomicrobia 疣微菌门,Fusobacteria 梭杆菌门
在我们体表与消化道内定植的细菌属于同一门,但门的丰度有所不同。这主要是因为皮肤和局部环境(氧气、酸度…)提供的营养促进了某些细菌的生长,而非其他细菌(图3)。
皮肤的微生物群主要由厚壁菌门、变形菌门和放线菌门等组成,其中包括一种著名的丙酸杆菌——痤疮丙酸杆菌。阴道腔主要包含厚壁菌门,其中乳酸杆菌的比率很高(女性高达70%)。这些细菌能够耐受阴道的酸性(女性阴道内的pH值为4.5),它们甚至能帮助阴道维持酸性,并耐受氧气。如果阴道生态位因此有利于乳酸杆菌的优势地位,那么它们占肠道微生物群的比例将不到1%。
肺部微生物群在2010年被描述。虽然人们早就知道致病菌可以在肺部创造一个生态位并诱发呼吸道疾病,但直到使用了当前的测序方法,人们才更好地了解健康个体肺部中天然存在的细菌。这种肺部微生物群在每克肺或肺叶支气管和肺泡中的液体中含有约10,000个细菌。主要由变形菌门、厚壁菌门和拟杆菌门组成。一个新的研究领域正在开辟,以更好地了解细菌对肺免疫的贡献和个体对某些呼吸道疾病的易感性。
因此,作为真正的细菌生态系统,多样的微生物群高度依赖于它们定植的区域,并对所有外部干预敏感。这就是为什么人体内微生物群的组成并非一成不变;它们构成的生态系统是动态的,并根据选择压力而演变。例如,月经会破坏阴道内不同乳酸杆菌之间的平衡。频繁使用肥皂会导致细菌种类的死亡率上升,促进酵母菌的增殖,并引起假丝酵母属(又称念珠菌属)真菌感染。食物,或者广义上我们摄入的所有产品,如药物(抗生素、抗酸剂……)都会改变口腔和肠道中的细菌。
2. 肠道生态系统
维持内稳态 一套维持平衡的生物调节机制,如调节体温和生理机能的生物活动是人体细胞、微生物群的活动及其不断相互作用的结果。例如,消化道的功能(消化、抵御病原体)由细胞和细菌共同提供。因此,肠道微生物群保证了大量对我们健康有益的生物功能。它与我们自身的细胞合作,帮助我们消化食物、预防感染、并促进我们获得性和先天性免疫系统的成熟。肠道微生物群还可以通过产生能够诱发生物反应的分子来促进肠外功能,它们还可以远程对大脑产生作用。
2.1. 一个生态系统的诞生
构成消化道的细菌和肠道细菌之间,彼此交换信号,相互容忍,有时还会争夺营养。因此,消化道必须被视为一个生态系统,其功能和结构取决于周围环境中细菌和细胞之间的相互作用以及物质和能量的流动。
肠道中细菌和细胞群之间的动态平衡是在人体出生时共同构建的,是渐进且连续的。出生时,我们的身体被认为没有微生物,而一旦与环境接触,定植就开始了。乳杆菌属(肠球菌)和肠杆菌科(大肠杆菌)的细菌以及双歧杆菌从出生就定植于消化道。第一批细菌被称为“初始定殖菌”,它们会降低氧气水平,这有助于诸如柔嫩梭菌这些对氧气过于敏感的细菌的定植。这些初始定殖细菌在围产期丰度高且占优势,在出生后2到3年后变为次优势菌。初始定植取决于许多因素,包括出生条件和婴儿的喂养习惯。例如,母乳中的细菌有助于儿童肠道微生物群的多样化;再如,剖腹产和自然分娩的婴儿肠道微生物群也有差异。在剖腹产情况下,儿童微生物群的组成接近环境中的细菌种群(医务人员的手、皮肤),而自然分娩的孩子的肠道微生物群则更接近母亲阴道内的微生物群。初始定殖菌在新生儿中发挥着重要作用,因为它们与宿主建立了第一次对话。出生时获得的微生物群会影响其他细菌的植入。
在生命的前三年,肠道微生物群在初始定植后形成,并会根据饮食中遇到的细菌、环境和孩子周围的人不断进化。然而,现在的生活方式,如抗生素的过度使用和生活环境的过度清洁,会让我们大量减少接触细菌的机会,这可能会延迟或不利于宿主与其微生物群之间建立共生关系。这种共生关系的改变可能会造成免疫系统成熟过程中的缺陷,增加诸如过敏性哮喘或炎症性疾病等过敏性疾病的发生。
2.2. 微生物群的细菌种群:一种平衡状态
我们已经通过许多例子证明了肠道微生物群的灵活性。微生物种群之间的平衡总是会根据生活条件和饮食而振荡。微生物种群动态也受到微生物自身的影响,它们可以是“照顾者或帮手”,也可能是竞争者或病原体。因此,发酵食品携带的微生物会暂时影响我们的肠道菌群。当你吃了100克酸奶,你会摄入大约10亿个细菌,它们属于两个物种:保加利亚乳杆菌和嗜热链球菌。即使这些由食物带来的微生物不会创建一个永久性的生态位,它们的传播也会改变微生物群的组成和活动。例如,通过产生乳酸,食源性细菌产生的乳酸可能有利于消耗乳酸的细菌的生长。
微生物群的平衡也可能受到严重干扰。当这种情况持续到现有平衡被打破时,就被称为“生态失调”。例如,在抗生素治疗或饮食突然改变后,会出现明显的生态失调。在日常生活中,饮食的改变会导致腹泻,这是肠道微生物群被严重破坏的一个明显表现。在大多数情况下,微生物群的变化是暂时的,并且与某些特定情况有关,因此微生物群受到中等应激时,可以找到一种与应激前接近的菌群结构,并迅速复原。
但在某些情况下,微生物群的组成将被永久性地破坏。在这种情况下,微生物群和宿主之间的互惠关系就不复存在了,它们之间也不再彼此适应。面对改造过的微生物群,肠道细胞不能充分反应,有时这会导致宿主配对/爆炸性微生物群引起身体的交替变化。这种微生物群和宿主不再处于共生关系的阶段就是生态失调,与虚弱状态或患病有关。例如,在患有慢性炎症的患者中,肠道微生物群的肠杆菌科(大肠杆菌)过多,而创始菌(如普拉梭菌)减少。在这些患者中,这种失调伴随着参与这种病理的肠黏膜炎症和肠屏障的改变。
2.3. 人体细胞和细菌参与消化
食物被我们细胞中的酶降解:脂肪酶、淀粉酶、蔗糖酶……。这些酶足以让我们吸收脂肪、消化蛋白质或淀粉,却不能消化所有食物。
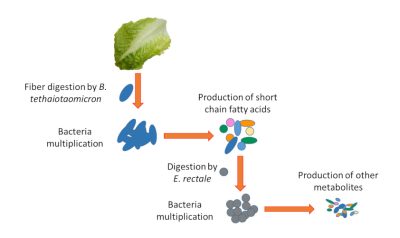
Fiber digestion by B. thetaiotaomicron 由多形拟杆菌消化纤维
Bacteria multiplication细菌增殖
Production of short chain fatty acids 产生短链脂肪酸
Digestion by E.rectale 由直肠真杆菌消化
Bacteria multiplication 细菌增殖
Production of other metabolites 产生其他代谢物
例如,我们的细胞不能消化由低聚多糖组成的植物纤维,而一些细菌有能够消化这些纤维的酶(如多形拟杆菌)。它们将这些由复合糖组成的纤维转化为我们身体可以使用的简单营养物质。其他细菌和我们的细胞一样,也不能消化纤维,因此需要依赖其他细菌(如直肠真杆菌)产生的简单营养物质。在这个阶段,我们可以看到生态系统的复杂性。如果因为没有摄入这些纤维,或消化它们的细菌(如多形拟杆菌)发生了改变,纤维将不再被消化,这会影响如直肠真杆菌这类细菌的存活(图4)。
营养物质在降解后会通过细胞膜上被称为“转运蛋白”的蛋白质被运输到肠道细胞中,再通过其他转运蛋白从肠道细胞进入血液。这些载体对营养物质在我们体内的输送至关重要,是由细菌和人体细胞共同控制的。例如,肠道细胞中的铁转运蛋白也部分依赖于肠道细菌。因此,细菌的任何改变都可能影响消化和营养物质在体内的输送。
最后,细菌不仅仅帮助我们消化。它们还能产生营养,包括维生素K等维生素。维生素K对血液凝固至关重要,如果没有维生素K,我们就会流血不止。
2.4. 微生物群/肠道生态系统:抵御入侵者的重要屏障
消化道位于外部环境和人体内部环境的界面处,作为一道屏障起着至关重要的作用。我们的细胞和我们所寄生的微生物共同保护我们的健康。细菌可以抵御入侵者并塑造我们的免疫系统,而我们的细胞则会形成一种选择性的细胞免疫屏障。
肠道微生物群的主要作用之一是防止病原微生物的增殖。因此,细菌密度对维持这一屏障至关重要。抗生素治疗是最广为人知的可能破坏这种平衡的一种情况。抗生素不仅能消灭引起感染的细菌,还能消灭所有对抗生素敏感的肠道细菌。因此,如果抗生素针对的是广谱细菌,即能够杀死来自完全不同种类的细菌,那么肠道微生物群就会发生更大的变化。这种对肠道细菌的大屠杀使得致病菌得以增殖。梭状芽孢杆菌感染是抗生素治疗可能引起的并发症之一。因此,抗生素治疗时间越长、抑菌谱越广,梭状芽孢杆菌感染的风险就越大。
肠道上皮细胞是位于肠道光面(微生物群被限制在此处)和宿主内部环境之间界面处的第一层细胞。在不断更新的过程中,上皮细胞由增殖的干细胞和参与营养吸收或分子分泌的分化细胞组成。增殖与分化之间的平衡对消化道的保护作用至关重要。上皮细胞的过度增殖是一种防御,可以帮助驱逐病原体。当上皮细胞屏障变得松散且过滤细菌或细菌碎片的效果较差时,就会出现生态失调。
肠上皮细胞还通过分泌黏液发挥重要的保护作用。通过排列上皮细胞,黏液类似于由复合糖蛋白组成的粘性凝胶{添加了糖的蛋白质,糖会赋予蛋白质特定的生物学特性。它们存在于细胞膜上,可以促进细胞间的相互作用}。 粘液是由肠上皮的特殊细胞产生和分泌的。这种粘液使细菌远离上皮细胞,防止它们与细胞直接相互作用。粘液提供的屏障可以通过食物和细菌自身来调节。能够消化膳食纤维的细菌也喜欢黏液中的复合糖,因此也是微生物群中某些细菌的营养来源。如果饮食中复合糖含量低,多形拟杆菌就会降解黏液;但作为补偿,它可以发出刺激黏液分泌的信号。这是菌群/肠道生态系统互利共生的好例子。
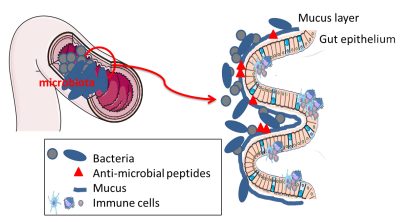
Microbiota微生物群; Mucus layer黏液层; Gut epithelium肠上皮细胞; Bacteria细菌; Anti-microbial peptides抗菌肽; Mucus黏液; Immune cells免疫细胞
除了由黏液施加的物理屏障外,一些肠细胞产生的具有抗菌作用的代谢物也会形成一道化学屏障。这些抗菌物质使细菌远离肠道细胞,避免任何侵入或不受控制的接触(图5)。
2.5. 培养我们免疫系统的细菌
我们的免疫系统是用来对抗感染的,无论是病毒、真菌还是细菌感染,但它也通过产生特定抗体来帮助控制肠道细菌。这些IgA 免疫球蛋白A,是免疫球蛋白(也称为抗体)的一种形式(或亚型)。免疫球蛋白是我们的免疫细胞(B淋巴细胞)对外来分子(可能是细菌)作出反应而产生的蛋白质,可以根据其作用分为不同亚型(A, G, M…)。进入消化道后,可以捕获细菌,并使它们通过粪便排出体外。
我们的身体在耐受细菌存在的同时,也随时准备对抗细菌的入侵。我们的细胞有细菌传感器,更准确地说,是细菌的某些成分。这些被称为受体的探测器会识别细菌模式,如因细菌而异的脂多糖含有脂质和碳水化合物的分子(LPS)[3]。当细胞检测到脂多糖时,它们会触发一系列连锁反应,激活免疫系统以控制感染。肠道微生物群中的细菌也不例外,它们也有LPS。另一方面,如果我们的探测器判断检测到的LPS不需要免疫应答,则不会清除产生LPS的细菌。因此,这种对肠道细菌的耐受性是一种脆弱的平衡,对微生物群的任何破坏都可能导致免疫系统的失调和异常激活。
细菌在训练免疫系统方面也起着至关重要的作用。事实上,在“无菌”动物(没有微生物群)体内,免疫系统是不成熟的。这证明我们自身的微生物群可以培养我们的免疫力。从微生物群中连续植入不同种类的细菌,对儿童早期免疫系统的成熟至关重要。破坏这种获取细菌物种的顺序会影响免疫系统的成熟,并导致某些过敏疾病。
因此,我们的消化道需要这些细菌,而这些细菌也必须使自己能够被耐受。然而,这些细菌对于教我们如何对抗病原菌也是必不可少的。
2.6. 微生物群/肠道细胞组合:对全身的影响
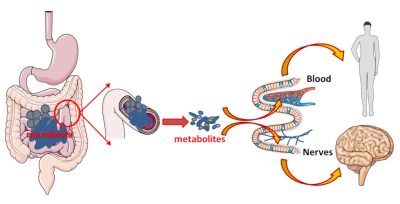
Microbiota微生物群;metabolites代谢物;blood血液;nerves神经
微生物群/肠道细胞组合可以有效帮助我们消化、吸收和提供营养;这对不可分割的组合也会产生信号,扩散至全身,并产生远端影响。这些信号由细菌、细菌代谢物或细菌碎片发出,通过肠细胞传送到血液,作用于我们的器官。这些代谢物也可以立即起作用。在这种情况下,细菌产生的代谢物会将神经连接丰富的肠道作为中介,通过激活这些神经连接来传递信息(图6)。
虽然微生物群/肠道生态系统仅存在于消化道,但它们不仅能够在局部发挥作用,还能够通过代谢物、神经网络和/或迷走神经{又称胃肺神经。有两条,分别支配身体的两侧。这些神经从脊髓顶端的延髓开始,并支配心脏、肺和肠道。信息的传递是无意识的(自主的)。}对远程发挥作用。因此,有一种观点认为,肠道菌群在脑-肠轴中扮演指挥的角色。
3. 肠道生态系统的破坏:疾病发展的原因
微生物群和宿主之间的对话和共生关系是脆弱的,肠道细菌组成或宿主的任何变化都可能破坏肠道生态系统。近年来,得益于高通量测序技术,研究表明许多疾病都与菌群失调有关,如慢性肠道炎症、肥胖、肝脏炎症(非病毒性肝炎)…… 但菌群失调也与心血管疾病、过敏和行为或精神性疾病(压力、抑郁、自闭症)有关。
因此,当前的主要问题之一是弄清菌群失调是这些疾病造成的结果还是其原因之一。人们已经认识到肠道微生物群是导致许多疾病发展的辅助因子;慢性炎症性肠道疾病、某些肝脏疾病、心血管疾病、某些过敏甚至自闭症等神经性疾病都是如此。
3.1. 微生物群和消化系统疾病
对肠道微生物群致病的研究首先针对的是消化系统疾病。一些细菌,如普拉梭菌,似乎在消化道的慢性炎症疾病中发挥重要作用,特别是在克罗恩氏病中。因此,重症或复发的患者体内具有最低水平的普拉梭菌。这种细菌产生的代谢物可以减轻炎症,从而减少癫痫发作。因此,目前的研究旨在培养这种细菌并破译其作用机制。然而,这种细菌对氧高度敏感,实现大规模生产来开发新疗法对研究人员提出了很大的技术挑战。
3.2. 微生物群和代谢综合症
代谢综合征是一系列与超重或肥胖相关的代谢改变,它会引起各种并发症,包括心血管疾病和糖尿病。与肥胖相关的并发症与肠道屏障的改变和细菌多样性的减少有关。此外,无论如何采用何种治疗方法,症状的改善都与更大的细菌多样性和肠道屏障的改善有关。
微生物群和肥胖。全球超重或肥胖人数的增加是由于暴饮暴食,但也与食物营养质量的变化有关。几百年前,纤维占我们日常饮食的近60%,而现在只占我们每日摄入量的10%左右。然而,这些变化并不总是能解释为什么有些人会更容易出现肥胖或相关并发症(心血管疾病、2型糖尿病{以慢性高血糖为特征,即血糖水平过高。调节血液中葡萄糖水平的胰岛素仍然会分泌,但不再起作用,即胰岛素抵抗})。鉴于肠道菌群的不同功能,特别是代谢功能,其在肥胖中的作用被广泛研究。
肥胖与肠道微生物群中细菌多样性的减少有关,因而也与细菌基因的减少有关。肥胖患者的微生物群移植到小鼠体内后,可以使小鼠肥胖。此外,在肥胖个体中,如果饮食中的细菌多样性高,则节食效果会更好。因此,微生物群会影响肥胖。另一方面,如果肠道细菌阿克曼菌和普拉梭菌与肠道微生物群的有益分布有关,它们则无法自行对抗生态失调。
心血管疾病和2型糖尿病。通常与肥胖相关的并发症包括心血管疾病和2型糖尿病。然而,并不是所有超重或肥胖的人都会出现这些并发症。除了基因遗传之外,肠道微生物群也会影响个体对这些并发症的易感性。
在2型糖尿病患者中观察到肠道微生物群的细菌组成发生变化。在这些患者中,某些细菌物种的数量减少显著,如普拉梭菌和罗斯氏菌属细菌。阿克曼菌也减少了;与肥胖患者一样,对啮齿动物施用阿克曼菌明显降低了其胰岛素抵抗。但是,肠道微生物群产生的代谢物与我们身体功能之间的相互作用才刚刚开始被破译,这表明宿主/细菌生态系统对某些疾病的影响是复杂的。
在代谢综合征的并发症中,心血管疾病至今仍是法国人死亡的首要原因。在这些病状中,动脉粥样硬化斑块 由各种成分(胆固醇、细胞碎片和钙)组成的聚积物,会落在动脉上,导致动脉堵塞。当斑块堵塞血管时,称为血栓形成。动脉粥样硬化是大多数心血管疾病的主要原因会破裂并形成阻塞血管的凝块。然而,肠道细菌在这些斑块的形成中发挥了作用。事实上,根据我们消化道中的细菌,某些食物的化合物会导致促进形成粥样斑块分子的形成(TMA 三甲胺然后被肝脏转化为TMAO 氧化三甲胺)。因此,食用含有胆碱 这些营养物质将被整合到我们体内不同的生物分子中。其中,卵磷脂是一种含有胆碱的磷脂。、卵磷脂或肉毒碱{非必需氨基酸}的肉制品有利于这种分子的形成。在这些斑块中也直接发现了细菌,其来源似乎更多是口腔而非肠道。
虽然富含纤维的地中海饮食对心血管疾病有益处,但尚未发现与这种有益影响有关的细菌。在啮齿动物研究中,作为益生菌的一些乳酸杆菌也显示出对心脏功能的积极影响。然而,肠道微生物群似乎在心血管并发症的发展中起到一定作用,但针对细菌的治疗方法还有待开发。
3.3. 微生物群和神经性疾病
肠道细菌和大脑之间的交流涉及到它们产生的各种代谢物。如2.6所述,这些代谢物通过血液或直接作用于消化道的神经末梢,在我们的神经元中发挥作用。此外,已有研究表明,肠道微生物群的性质会影响血脑屏障的严密性;这道屏障通过限制可能对大脑功能产生负面影响的物质来保护我们的大脑。
最新研究表明,肠道细菌的性质会影响焦虑和压力的状态,对抑郁症也有影响。在啮齿动物中,补充乳酸杆菌或双歧杆菌(特别是婴儿双歧杆菌)似乎可以改善抑郁症状。虽然有研究发现,在抑郁期间某些细菌水平较低(如普拉梭菌),但目前尚不清楚这种下降是否直接导致抑郁症状。
生态失调也与一些神经退行性疾病(帕金森病、阿尔茨海默病和多发性硬化症)相关。 然而,目前尚不清楚生态失调在多大程度上伴随并加剧这些疾病。
4. 微生物群,未来的治疗靶点
恢复肠道/微生物群生态系统的平衡是开发新疗法的一大挑战。与此同时,食物已经是影响微生物群的巨大杠杆。例如,在饮食中摄取足够的纤维有助于促进大量细菌的生长。我们也可以通过发酵食物或富含细菌的食物补充剂,在某些疾病的治疗中引入缺失或不足的细菌。在这些纤维中,有些纤维已经通过调节肠道微生物群证实了它们对宿主的有益作用,这些就是益生元。
对我们的健康有益的肠道细菌可以作为食物补充剂来食用,这可以弥补它们在某些疾病中的缺失或下降。例如,普拉梭菌在健康个体中占主导地位…… 但在许多疾病中存在量较少,包括慢性炎症性肠病(IBD)。
在医疗规程中使用肠道菌群已经成为医院的标准做法。例如,治疗对常规抗生素治疗有耐药性的复发性梭状芽孢梭菌感染可以采用粪菌移植,将健康捐赠者的粪便菌群移植到接受者的肠道中。经过这种粪菌转移,患者将不再遭受梭状芽孢梭菌感染的困扰。因此,微生物生态系统已经被用作一种治疗策略,这种粪菌转移技术可能会应用到其他病症的治疗中。
这些都是基于恢复肠道/微生物群生态系统平衡的治疗策略,对我们身体的正常运作至关重要[4]。
参考资料及说明
封面图片:由Teppei Ikehila绘制:Teppei Ikehila被神奇的细菌、能够无限复制的小细胞、奇特的形状和颜色所吸引,使它成为一个特别鼓舞人心的主题。
[1] 参考举例:人类微生物组计划 (http://hmpdacc.org/), Metahit (http://www.metahit.eu/)
[2] 在反刍动物中,古细菌可占胃或瘤胃中细菌的3% 至 3.3%。在人类中,肠道微生物群中古菌的存在似乎不是系统性的。然而,可能存在偏差,因为许多研究没有将古菌包括在他们的研究中。
[3] 当这些受体出现在细胞膜上时,它们是toll样受体或TLR受体。它们也可以存在于细胞中,并识别肽聚糖类型的细菌模式。这些是NOD接收器。
[4] Kapel et al (2014) Practical implementation of fecal transplantation. Clin Microbiol Infect. 20(11):1098-1105; French Group of Faecal microbiota Transplantation (FGFT) (2016) Faecal microbiota transplantation in recurrent Clostridium difficile infection: Recommendations from the French Group of Faecal microbiota Transplantation. Sokol Dig Liver Dis. 48(3):242-247.
环境百科全书由环境和能源百科全书协会出版 (www.a3e.fr),该协会与格勒诺布尔阿尔卑斯大学和格勒诺布尔INP有合同关系,并由法国科学院赞助。
引用这篇文章: CASSARD Anne-Marie, THOMAS Muriel (2024年3月9日), 人类微生物群:我们健康的盟友, 环境百科全书,咨询于 2025年1月7日 [在线ISSN 2555-0950]网址: https://www.encyclopedie-environnement.org/zh/sante-zh/human-microbiotas-allies-for-our-health/.
环境百科全书中的文章是根据知识共享BY-NC-SA许可条款提供的,该许可授权复制的条件是:引用来源,不作商业使用,共享相同的初始条件,并且在每次重复使用或分发时复制知识共享BY-NC-SA许可声明。