Mercury, fish and gold miners
PDF
Mercury is a good example of the difficulties in assessing and managing an environmental health risk. The presence of this metal in the food chain has a demonstrated potential risk, which must be weighed against the benefits of consuming certain foods such as fish. The history of mercury and its health effects, often marked by dramatic events, has illustrated progress in environmental health for decades.
1. Mercury

Mercury exists schematically in different forms: elemental mercury or metal mercury, inorganic mercury salts and organic mercury [1]. It is the properties of mercury metal that have long made mercury successful in the industry. Relatively chemically inert, the only metal that is liquid and volatile at room temperature, it is capable of dissolving many metals to form amalgams [2].
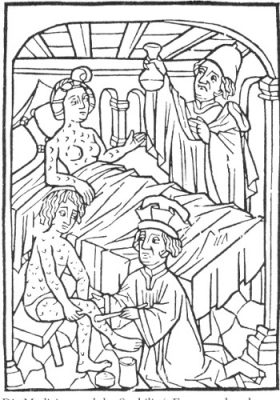
“Inventor” of Occupational Medicine by observing pulmonary pathologies in miners, he was the first to introduce mercury into therapy, particularly in the treatment of syphilis (Figure 2). When he noticed the many adverse effects of mercury, he had this phrase that has remained famous in toxicology: “it is the dose that makes the poison”. And the popular saying was then “a night with Venus, a life with Mercury”!
1.1. Mercury metal
The toxicity of mercury metal to humans comes from its high volatility at room temperature, it is the “vapour” risk. This is why any presence of metal mercury in “broad public” devices (thermometer, barometer, etc.) was banned in France in 1999. This is why no more high school student sees his teacher arriving with a few mercury balls rolled in his hand (Figure 3): the older ones could remember this. Ingested, metal mercury does not pass through the digestive barrier and therefore is not absorbed into the body. In other words, mercury in this form no longer represents a public health issue. But metal mercury will still be present in this article.


1.2. Inorganic mercury
Again and apart from a few individual accidents, there is no real public health issue with this form of mercury. It is still encountered as inorganic mercury salts in some laboratories, in some button cells, but nothing very important for human health. But this does not prevent the active search for substitutes for certain industrial products such as button batteries, for example.
1.3. Organic mercury
The main issue of mercury lies here, in the general population [3],[4]! As we will see in details, it is this organic mercury to which humans are often exposed through their diet. In an almost “unstoppable” and unfortunately almost “definitive” way, for which man is largely responsible. Until 1989, organic mercury was used as a fungicide to destroy microscopic fungi present in cereals in particular: we will see the dramatic consequences. Toxic to the central nervous system, it has caused serious neurological complications in exposed individuals. As the problem is now well identified, surveillance systems for exposed populations have been set up by Santé Publique France [5] and health recommendations are regularly published by ANSES [6].
2. Organic mercury, a public health issue
A few dramatic events have marked the history of organic mercury and played an essential role in a better understanding of its toxicity and its incorporation into the food chain.
2.1. Minamata Bay
A real police thriller took place in the 1950s in Japan. The story begins with the appearance of severe neurological pathologies in fishing families living along the bay. What we would call today “clusters” of patients, i.e. patients who are well grouped in the same region.
Adults had severe paralysis, neuropsychic disorders; many children had severe malformations with very disabling neurological and psychological disorders, if not stillborn. These facts took place over several years and despite many expert opinions, the mystery remained total! The final toll will be about 600 dead and 3,000 sick! At that time, epidemiology was still a nascent science and toxicological analyses of biological fluids (blood, urine, etc.) were still quite rudimentary. We must imagine this region of Japan affected for several years by a mysterious disease whose cause no one understands.
A few clues will gradually be identified, two in particular: these families feed mainly on fishery products and the very large chemical company Chisso, which uses mercury as a catalyst for certain chemical syntheses, regularly discharges its effluents into the waters of this Pacific Bay.
As the story goes, toxicologists from Scotland found out what was going on eventually. How? First, they looked through all the patients’ medical records to find possible commonalities. Their conclusion: the pathologies described were similar to what was then known about the toxicity of organic mercury. However, the Chisso plant mostly dumped metal mercury into the Pacific! As we have seen, only the vapours of mercury metal are toxic to humans and it does not pass through the digestive barrier when ingested. The mystery persisted for some time.
Stubbornly, the experts ended up making the link between the Chisso factory, which they suspected of playing a role in this “epidemic”, and the pathologies presented by the fishing families.
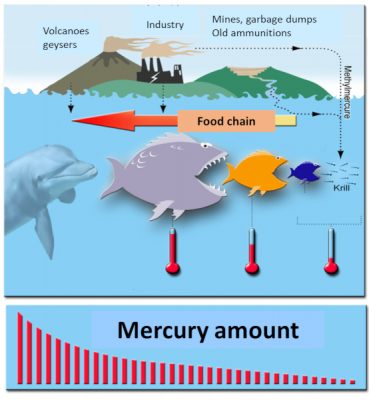
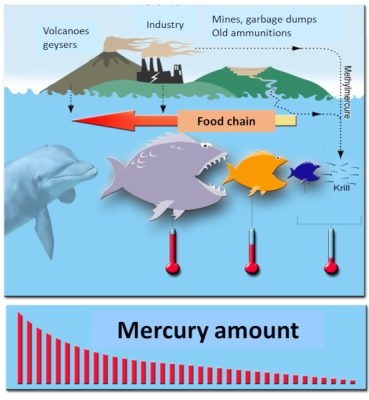
Another phenomenon now well known in environmental health was also highlighted, bioaccumulation. Mercury is a “cumulative” toxic that accumulates in organisms over time: the older the fish or mammal, the higher its organic mercury content. Another phenomenon has been described, bioamplification: large fish eat small fish, organic mercury accumulates throughout the food chain to humans: being at the end of the chain, humans then receive the maximum dose of organic mercury when they eat swordfish, tuna and halibut for example. Or other large fish (Figure 5).

2.2. Fishermen and ice cores
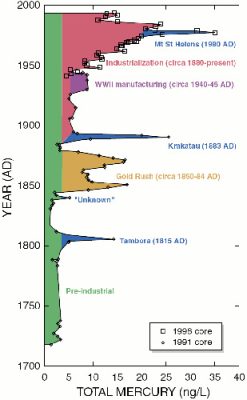
Intrigued by the history of Minamata Bay, some scientists asked themselves the following question: is it an isolated industrial accident? Or are the seas and oceans already polluted by mercury as a result of the industrial revolution that emerged at the end of the 19th century? First, it should be noted that mercury present in the mammalian body is partially eliminated by the appendages, i.e. hair and nails in humans, and feathers in birds. As it is usual to proceed in Science since Claude Bernard, these scientists put forward a hypothesis: the seas and oceans are not contaminated, it was an isolated industrial accident in Minamata.

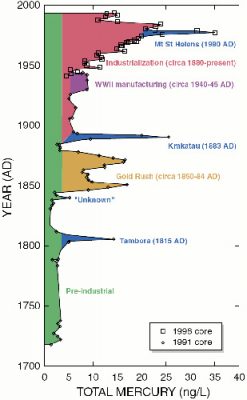
More recently, it has been shown that permafrost (frozen ground in the northern hemisphere) contains huge amounts of mercury [10]; global warming may raise fears of a gradual release of this mercury.
2.3. A famine in Iraq
A terrible famine appeared in the Iraqi countryside in the 1970s. To help the populations, very large quantities of cereals were then distributed in bags, by the State and also by Associations (which were not yet called NGOs). Quite quickly, various neurological disorders appeared in these farmers and their families. We remember that methyl mercury was then used as a fungicide on cereals. Indeed, cereals had been treated with methyl mercury to use them as seeds but not for immediate consumption. However, on the bags, the inscriptions were labelled in English, a language of which these populations had no knowledge! It was therefore well after the consumption of these treated cereals that these intoxications appeared. Fortunately, almost 20 years after Minamata, the link was quickly established between methyl mercury and the pathologies presented by these populations. The final result was nevertheless dramatic, with nearly 650 deaths and 6,500 patients [11].
This tragedy nevertheless allowed progress to be made. The dose-response relationships of mercury have been better studied and since then hair toxicology has become a standard in the study of chronic heavy metal poisoning.
2.4. The Faroe Islands, Seychelles
In order to improve our understanding of human exposure to organic mercury and its toxicity, numerous studies are being conducted to monitor exposed populations and assess risks. For example, in the Indian Ocean, a monitoring of groups of children from the Faroe Islands and Seychelles was established in 1985 [12]. These populations, which consume a lot of fish, are indeed “good witnesses” of possible chronic exposure to low doses of methyl mercury. Thus, progress can be made thanks to these studies, including the use of fine cognitive tests to detect the smallest signs of toxicity or the determination of mercury in the cord blood from birth.
Nevertheless, it should be noted that these epidemiological studies, known as “cohort” studies, are extremely difficult. Many biases may occur throughout the study. For example, the fact that fish may unfortunately contain other pollutants or that other environmental factors may be present throughout the life of the subjects being monitored. It is therefore difficult to attribute all observations to mercury alone. This is a well-known and common fact in all targeted studies on the effects of the environment on human health.
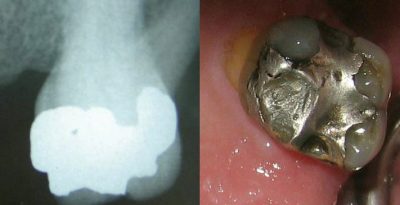
3. Dental amalgams
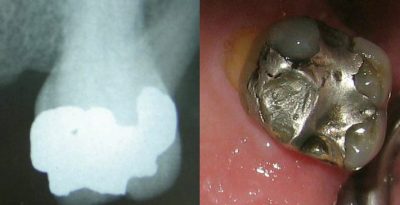
The mercury contained in these amalgams is believed to be responsible for various chronic diseases such as Alzheimer’s disease or multiple sclerosis. Mercury vapours can be inhaled and thus enter the body: chewing gum and bruxism [13] increase the risk of mercury vapours being released. To date, no national or international scientific expertise has been able to establish a formal relationship between the presence of amalgams in the mouth and these diseases.
But passions are strong as soon as we talk about it! However, we can regret some of the consequences: the business done in European countries where health professionals claim to cure patients by administering mercury chelating products at a high price. We will see below that it is very difficult to manage this type of risk that is not formally proven.
4. Mercury and gold panners

But above all, the disaster associated with these gold panning practices lies in the fact that all the rivers in this large Amerindian zone are massively polluted by mercury metal (Figure 10). All populations in this area, which covers French Guiana, part of Brazil and Suriname, are exposed to methylmercury, with mercury metal from gold panners being transformed into methylmercury by aquatic micro-organisms. Santé Publique France, the health agency in charge of population monitoring, has long since set up population monitoring measures in this department and regularly communicates with ANSES on the risks of excessive fish consumption (see refs. [1] and [2]).
5. Mercury and risk management
Mercury is an excellent example of the difficulty of managing an environmental health risk. Risk management follows risk assessment.
Risk assessment is carried out by independent scientists: they make proposals based on the current state of knowledge and indicate the health risks of exposure to a particular toxic substance; they also usually propose exposure values that should not be exceeded.
Risk management is the responsibility of the decision-maker: it is the role in France of our health agencies, here the ANSES, and of our government ministers concerned with the subject. These decision-makers may or may not rely on the conclusions of scientific experts. We will take two examples.
- What are the recommendations for fish consumption?
The difficulty is compounded by the fact that fish can be contaminated with many pollutants other than mercury, PCBs and dioxins, among others. Risk management will always seek a balance between – you have to eat fish – and – be careful of the toxic substances contained in fish. We know the richness of fish in certain nutrients that are precious for health, such as Omega 3 from fatty fish. We also now know the main contaminants in fish and their health risks (PCBs, dioxins, heavy metals including mercury). The recommendations are then “protective” without being able to formally prove their value. The ANSES thus limits the consumption of fish by young children and pregnant women [14]. - What about dental amalgams?
They pose another problem, that of substitutes, in this case the resins most often used in dental surgery. Can it be shown to be completely safe? No, not definitely. Concerning the presence of mercury itself, the recent recommendations are again “protective”, according to the ANSM (Agence nationale de sécurité du médicament et des produits de santé) of the precautionary principle and despite the absence of proven risk according to all recent studies: avoid mercury amalgams in young children and pregnant women [15]. On the other hand, and to be complete, it should be pointed out that the use of mercury in dental offices has been subject to very strict regulations for many years, in particular to avoid the risk of repeated toxic inhalation by the dental surgeon and, more occasionally, by the patient. Recommendations for patients [16] and professionals [17] have been proposed by the ANSM [18].
The mismanaged use of a large number of heavy metals such as mercury, but also lead, arsenic or cadmium, has led to massive and sustainable pollution of the planet for centuries. Unfortunately, these metals all have proven harmful effects on human health.
Mercury represents a real public health issue, in the general population, in its organic form, methyl mercury in particular. As we have seen, it enters the food chain when eating seafood, especially fish. Let us also remember that we must eat fish, but not too much and not every day. Risk management is a difficult exercise when, as in this case, it is necessary to find a balance between what is good and what is bad.
Mercury metal is not to be outdone even if its ingestion does not pose any risk. The identification of the phenomenon of biomethylation in the marine environment has clearly shown that environmental pollution of mercury metal is responsible for the incorporation of organic mercury into the food chain.
The case of dental amalgams is also a good example of the difficulty of managing a risk. Which one exists? Which doesn’t exist? Rightly or wrongly, the precautionary principle will surely find many applications here (see focus The precautionary principle).
Finally, an international convention on mercury has recently been implemented by the United Nations Environment Programme, the Minamata Convention. Adopted in 2013 in Japan, it entered into force on 16 August 2017 [19]. Among its main provisions, the Minamata Convention provides, inter alia, for the prohibition of new mercury mines and the phasing out of existing mines, the elimination and phasing out of the use of mercury in a number of products and processes, the establishment of measures to control emissions of mercury into the atmosphere and its releases to water and land, and the control of the informal artisanal and small-scale gold mining sector. The Convention also addresses the issue of interim storage of mercury and its disposal once it has become waste, contaminated sites and health aspects.
A very interesting video highlights global mercury pollution and puts this convention in perspective [20] :
6. Messages to remember
- Mercury is an excellent example of the difficulty of managing an environmental health risk that involves two steps:
- Risk assessment is carried out by independent scientists: their proposals are based on the current state of knowledge; they indicate the health risks of exposure to a particular toxic substance; they also propose exposure values that should not be exceeded.
- Risk management is the responsibility of the decision-maker: it is the role of the health agencies concerned by the subject. These decision-makers may or may not decide to rely on the conclusions of scientific experts.
- The toxicity of mercury metal to humans comes from its high volatility at room temperature. Occupational mercury poisoning was registered on the French Occupational Disease Register as early as 1919.
- Mercury represents a real public health issue, in its organic form, methyl mercury in particular.
- The identification of the phenomenon of biomethylation in the marine environment has shown that environmental pollution of mercury metal is responsible for the incorporation of organic mercury into the food chain.
- Mercury is a “cumulative” toxic that accumulates in organisms over time: it is called bioaccumulation.
- Biomagnification is another important process in environmental health: organic mercury accumulates throughout the food chain to humans: being at the end of the chain, humans receive the maximum dose of organic mercury when they eat swordfish, tuna or halibut, for example.
- Risk management will always seek a balance between – eating fish is good for your health – and – being careful about the toxic substances in fish.
- The property of mercury metal to amalgamate with other metals has long been used to form “fillings” made to fill dental caries.
- To date, no scientific expertise has been able to demonstrate any health risks potentially associated with the presence of dental amalgams.
- The recommendations are “protective” in application – according to the National Agency for the Safety of Medicines and Health Products – of the precautionary principle.
- The use of mercury in dental offices has been subject to very strict regulations for many years.
- Clandestine gold miners use mercury to recover any gold that may be present in river water. As a result, the rivers where this practice exists (French Guiana, for example) are massively polluted by mercury, creating irreparable environmental damage. In addition, there are health risks for populations associated with excessive fish consumption.
- The mismanaged use of a large number of heavy metals such as mercury, but also lead, arsenic or cadmium, has led to massive and sustainable pollution of the planet for centuries. Unfortunately, these metals all have proven harmful effects on human health.
Notes and references
Cover image. [Source: By Arnaud 25[CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0)], from Wikimedia Commons]
[1] Bensefa-Colas L, Andujar P, Descatha A (2011). Intoxication au mercure. La revue de Médecine Interne, 32 (7), pp.416-24.
[2] Assembly of heterogeneous elements. The word is most often used in chemistry to refer to a mercury alloy with other metals.
[3] In Environmental Health, a clear distinction is made between the population of professionals exposed to certain risks at the workplace and the general population, i.e. everyone outside the workplace.
[4] Clarkson TW (2002). The three modern faces of mercury. Environment Health Perspectives, 110 Suppl 1: 11-23.
[5] Santé Publique France : www.santepubliquefrance.fr
[6] YEARS. Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail. : www.anses.fr/fr
[7] Harada M (1995) Minamata Disease: Methylmercury poisoning in Japan caused by environmental pollution, Critical Reviews in Toxicology, 25:1, 1-24
[8] Bond AL, Hobson KA, Branfireun BA. 2015 Rapidly increasing methyl mercury in endangered ivory gull (Pagophila eburnea) feathers over a 130 year record. Procedings of the Royal Society of Biology, 282: 2015
[9] Schuster PF, Krabbenhoft DP, Naftz DL. 2001. Atmospheric mercury deposition during the last 270 years: a glacial ice core record of natural and anthropogenic sources. Environmental Science and Technology, 36, 2303-2310
[10] Shuster PF et al (2018). Permafrost stores a globally significant amount of mercury. Geophysical Research Letters, 45, 1-9
[11] Al-Tikriti K, Al-Mufti AW (1976). An outbreak of organomercury poisoning among Iraqi farmers. World Health Organization Bulletin. 53 Suppl:15-21
[12] Myers GJ, Davidson PW, Cox C and others (1995). Summary of the Seychelles child development study on the relationship of fetal methylmercury exposure to neurodevelopment. Neurotoxicology 16, 711-716.
[13] Repeated and unconscious tooth friction movements
[14] YEARS: Eating fish, benefits and risks. www.anses.fr/fr/content/manger-du-poisson-bénéfices-et-risques
[15] ANSM : Mercury in dental amalgams, data update, April 2015. http://ansm.sante.fr/content/download/76933/976017/version/1/file/ANSM_Report_Mercury-Amalgames-Dentaires_May-2015.pdf
[16] ANSM : http://ansm.sante.fr/var/ansm_site/storage/original/application/0b5892a526480ba401d0ccc3fd4ee0e2.pdf
[17] http://ansm.sante.fr/var/ansm_site/storage/original/original/application/7cacb0593aaa9f8ebd9b176c65ff98890.pdf
[18] ANSM : Agence nationale de sécurité du médicament et des produits de santé: http://ansm.sante.fr/
[19] Minamata Convention on Mercury: http://www.mercuryconvention.org/Portals/11/documents/Booklets/COP1%20version/Minamata-Convention-booklet-fr-full.pdf
[20] Make mercury history: https://youtu.be/guO7RbArWf4
The Encyclopedia of the Environment by the Association des Encyclopédies de l'Environnement et de l'Énergie (www.a3e.fr), contractually linked to the University of Grenoble Alpes and Grenoble INP, and sponsored by the French Academy of Sciences.
To cite this article: DANEL Vincent (August 16, 2019), Mercury, fish and gold miners, Encyclopedia of the Environment, Accessed December 22, 2024 [online ISSN 2555-0950] url : https://www.encyclopedie-environnement.org/en/health/mercury-fish-gold-miners/.
The articles in the Encyclopedia of the Environment are made available under the terms of the Creative Commons BY-NC-SA license, which authorizes reproduction subject to: citing the source, not making commercial use of them, sharing identical initial conditions, reproducing at each reuse or distribution the mention of this Creative Commons BY-NC-SA license.