鸟类如何适应气候变化?
作为一只鸟,要如何适应不可逆转的气候变暖呢?有三种解决方法:逃生到更适合的环境、适应本地环境、面临灭绝。最常见的机制就是所谓的“生境追踪”:鸟类会根据它们的温度适应范围,选择合适的纬度和高度生存环境。另一种机制是当温度上升得不是很快时,鸟类会逐渐适应这种温度的升高。但是最安全的机制是通过微进化,例如改变种群的基因结构来实现适应。当这些机制由于一些原因都不可行时,灭绝将不可避免。生活在极端环境中(如苔原或者高山)的物种十分容易灭绝,因为其栖息地的活动受到地理环境的限制。某些鸟类,尤其是长距离迁徙且在旅途中频繁改变栖息地类别的种类,一般不会受到新环境的影响。
1. 巨大的挑战
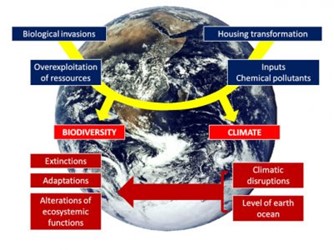
[图片来源:Jacques Blondel(雅克·布隆德尔)] Biological invasions(生物入侵) Overexploitation of resources(资源的过度开发), Inputs chemical pollutants(化学污染物输入), Housing transformation(栖息地迁移); BIODIVERSITY(生物多样性), CLIMATE(气候); Extinctions(灭绝), Adaptations(适应), Alterations of ecosystemic functions(生态系统功能转化); Climatic disruptions(气候混乱), Level of earth ocean(地球海平面)
气候变化已是毋庸置疑的事实,也是“全球变化”六大组成要素中的一部分,其他方面分别是栖息地改变、化学物质输入、自然资源过度开采、生物入侵(图1)。之所以使用“全球”一词,是因为它们各自的特点与全球息息相关;它们的影响也大多会波及全球(见《生物多样性不是奢侈品而是必需品》)。气候变化包括六种现象:二氧化碳浓度升高、温度升高、全球海平面上升、极地冰雪融化、干旱和沙漠地区扩大、极端事件增加(热浪、洪水、风暴和飓风)。这些剧变是地球人类中心化的征兆[1],并且从局部到全球,生物历史的方方面面都会受到影响[2]。
这种变化既迅速又突然,以至于让我们不得不面临一系列“自然实验”,这些实验展现出的一系列挑战,都是生物种群在面临全新环境时要应对的。新环境可能会导致生物灭绝,但是生物体也可以对这些环境变化作出响应(或者不响应),来赢得新的机会(见《生命对环境限制的适应》)。关于鸟类的脆弱性以及它们对全球变暖的适应性,人们已经进行了大量研究。据估计,鸟类会受到巨大影响[3],全球温度每升高1摄氏度,100–500种鸟类将面临灭绝[4]。
2. 温度是生理学和生态学的关键因素
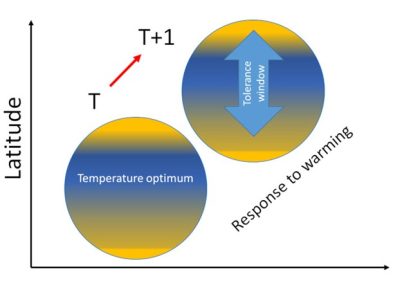
[图片来源:Jacques Blondel(雅克·布隆德尔)]。Latitude(纬度); Temperature optimum(最适温度); Response to warming(对全球变暖的响应); Tolerance window(耐受范围)
根据生态学的代谢原理,有机体的代谢谱控制着个体、种群和生态系统各个水平的生理和生态过程[5]。将生物身体质量与代谢速率分别取对数后,该宏观生态常数与物种尺寸大小存在线性关系,并且在大型生物和微生物中都适用。因为鸟类对于温度变化敏感,我们便可以由此进一步定义每个物种的温度耐受阈值,该阈值定义了对应物种可以适应的温度变化范围(图2)。跨越阈值上限或下限都会引起与一系列与代谢特征相关的形态学、生理学和数量统计学上的级联效应。这些效应便是有机体生物地理分布的根本原因。
生物体对全球变暖的响应表明生命从某一特定尺度水平到下一水平的效应是级联的:
- 在个体水平,温度影响生长、大小、繁殖,进而影响个体的适应能力;
- 之后个体水平的响应通过其年龄结构、密度和多样性(包括表型多样性和基因多样性)等转化为种群水平的响应。
- 接着,通过影响种群的组成、结构、能量生产、特异多样性以及动态,其响应会在物种集合的水平完成进一步转化。
- 最终,发生在种群水平上的响应会决定生态系统的功能。
许多实例都阐明了生物,尤其是鸟类,对温度变化的脆弱性。例如温度达到其温度耐受阈值上限后超出该值,或者温度变化导致生态系统生产力下降,以及温度变化扰乱物种间相互关系而导致食物网紊乱。
3. 鸟类对全球变暖的响应如何?
鸟类对全球变暖的响应主要有两种类型。第一种是以表型为本质的即时响应,即鸟类会根据温度变化而立即调整其行为(见《生命对环境限制的适应》)。即时响应可能是因为每一种生命历史特征都存在“表型可塑性”,即所谓的反应规范(见《顺应或调整》),它使得机体能够迅速对环境状态做出反应。第二种响应是比较难以证实的针对新选择机制的适应性反应,是一种微进化适应,也是一种以基因为本质的响应,从而适应环境产生的新选择压力。表型响应是迅速的,而微进化响应是跨世代的,因为它需要经过定向选择,筛选出后代遗传变异体中的有利变异,比如针对产卵时间或迁徙出发策略方面的变异。
3.1 “生境追踪”
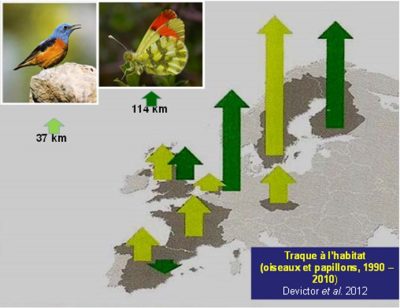
[图片来源:Jacques Blondel(雅克·布隆德尔)]。Traque a I’habitat(生境追踪); O is eaux et papillons, 1990-2010(鸟类和蝴蝶,1990-2010年)
向高纬度和高海拔移动,以适应温度阈值的变化,这似乎是预期中符合逻辑的响应。事实上,气候变化造成的最直接可见的结果是分布区的变化。在较大的时间和空间范围内,科学家已经证实,在更新世植被带及其动物群落的起伏变化是对冰期和间冰期交替的响应,这种交替在过去的两百万年间以大约100,000年的频率出现[6]。目前科学家已经利用古生物地理学和系统发生生物地理学方法测定了生物的迁徙路径和速度。这一基因方法让我们得以标记动物的线粒体DNA和植物的叶绿体DNA,从而在时间和空间上追踪携带单倍型分子标记的生物。
对于鸟类和蝴蝶这两类受试陆生物种而言,其大部分种类对当今的全球变暖都着显著的向高纬度迁移的适应变化[7],[8](图3)。20世纪90年代以来,非洲的500种鸟类中有200种的栖息地纬度上移[9],这一点已经在很大程度上得以证实,并且该趋势在21世纪也进一步加快。但是,尽管气候变暖对观察和测量的运动影响重大,我们也要特别注意气候与其他环境因素(尤其是栖息地的变化)之间的平衡。
然而,针对相关数据的任何解释都会存在其不确定性,因为生物的分布范围发生变化时,可能并不总会有对应的相关证据伴随出现。许多分布区的变化也不能被解释为对气候变化的响应。
因此,证实温度升高对生物迁移变化产生影响非常重要。
- 怎样测量生物对气候变暖响应中的迁移程度和速度?
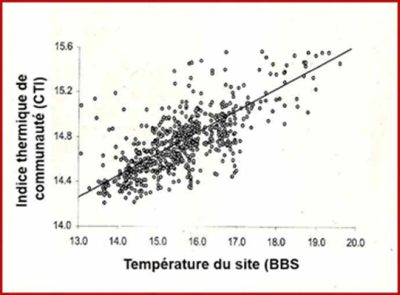
[图片来源:Jacques Blondel(雅克·布隆德尔)]。Indice thermique communaute(群落温度指数,CTI); Temperature du site(样点温度,BBS)
基于利用温度来预测鸟类分布范围的鲁棒性,德维克特(Devictor)等[10]使用由法国国家自然历史博物馆开发的STOC(Suivi Temporel des Oiseaux Communs,即鸟类临时监测)程序的数据包,该程序可以测定每年在相同地点繁殖的物种,从而检验了105种鸟类对气候变暖的响应。为此,他们计算了每个物种的分布区从三月到八月的平均温度(STI,物种温度指数)。然后,他们对在法国领土内给定点记录的每个物种集合中所有物种的温度指数取平均值,从而获得群落温度指数(CTI)。根据法国都市内记录的722个物种在当地鸟类群落中的丰度,加权计算出构成其群落物种的STI,再求其平均值获得CTI。CTI值可以表示局域种群内物种的平均“嗜热性”程度,CTI值高说明种群内包含许多喜温物种并且该地点的温度较高(图4,[10])。
这些数据让研究者们能够量化鸟类群落对全球变暖的响应:一定时间内当地种群的CTI值的升高表明种群中包含的喜温性物种数量增加(如生活在更南方的物种),或者换句话说,被喜温性物种取代的南方物种更少,因为南方物种已经向更北方移动。在对种群调查的地方,测定当地植被的CTI值,该值随时间的变化即可提供每个物种集合的移动信息。
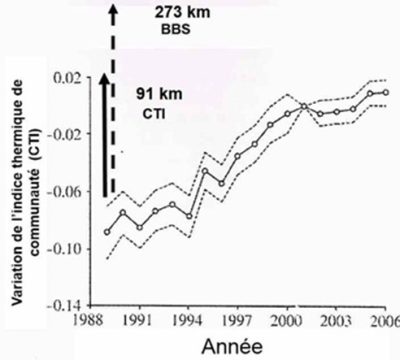
[图片来源:Jacques Blondel(雅克·布隆德尔)]。 de I’ indice thermique de communaute(群阔温度指数CTI的变化); Annee(年份)
通过计算722个地点,科学家在1961年至1990年之间每5年时间间隔内(3月至8月)内的CTI值,对该地环境温度进行了相同的实验。将局部气候变化投影到法国气候图(等温图)上,以便比较CTI和当地温度在其各自梯度上的位置,进而测量它们各自的位移速度。科学家还计算了从1989年到2006年间,在2×2km的网格上分布的法国722个测定点的CTI值的空间变异,结果与期望一致,每个点的CTI 值随时间变化而稳定增长,反映出法国当地群落的种群发生了变化,喜温物种富集,(南部物种逐渐北移),其平均位移为91±11km,与此同时,当地气温上升,等温线也相应北移了273km(图5,[7])。作者总结到,正如预期的那样,鸟类会向北迁徙以应对气候变暖,但它们迁徙的速度比预期要慢,因为这两个时期鸟类飞行的距离与等温线之间的距离相差182公里
由于许多未知的原因,不同物种的生境追踪移动结果有很大的差异,因此,任意栖息地的本地物种组合都会随时间变化。通过定期观察鸟类栖息地的纬度变化,生境追踪也可以反应鸟类栖息地沿着山坡发生的海拔高度变化。
- 极端环境下的情况又如何呢?

[图片来源:Jacques Blondel(雅克·布隆德尔); 照片来源:Jean-François Desmet(让-弗朗索瓦·德斯梅特)/保留版权]。BEFORE(之前); AFTER(之后)
当生物不能尽可能地“黏附”在其适宜生存的温度范围生境内时,例如在北极、南极或者高山等极端环境中繁殖的鸟类种群,如果它们没有做出适应性反应,其数量将面临骤减的风险。如图6所示,岩雷鸟(Lagopus muta)种群由于气候变暖,不断向山顶迁移,导致栖息地逐年缩小。在过去的三四十年中,北极冰区的面积以每年45000km²的速度融化。这样触目惊心的数字也出现在其他现象中,例如永久冻土的融化,亚森林的向北上移,苔原生境的缩小。为了应对气候变暖,在纬度和高度上的森林面积扩展会导致许多鹅和滨鸟物种特有的生境萎缩,估计到21世纪末将减少40%至57%,其中一半可能可能在本世纪末消失[11]。
3.2 气候变暖和鸟类迁徙:新的挑战
在过去的20年中,由于一些限制因素,例如迁移条件的恶化,越冬栖息地的破碎化和沙漠化,以及撒哈拉沙漠的不断扩大等,导致跨越撒哈拉的物种以每年1%的速度稳定下降[12]。
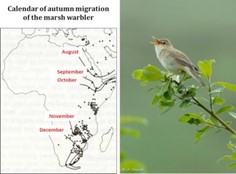
[图片来源:Jacques Blondel(雅克·布隆德尔); 照片来源:Jean-François Desmet(让-弗朗索瓦·德斯梅特/保留版权]。Calendar of autumn migration of the marsh warbler(湿地苇莺秋季迁徙月历); August(八月); September(九月); October (十月); November (十一月); December (十二月)
迁徙(图7)是一种复杂的现象,由复杂的行为、生理和内分泌适应决定。迁徙过程的时机严格,它也意味着,鸟类迁徙途中,其定期停留的不同地点都存在着相应资源储备,能够帮助其恢复体力。气候变化正在扰乱着进化过程中由不同因素形成的微妙的平衡。
苇莺家族是研究这些问题的良好模型,因为共有17个苇莺物种在欧洲繁殖,它们当中的一些跨撒哈拉沙漠迁徙,一些只部分迁徙,还有一些是不迁徙的。一项不同物种对气候变暖的响应模拟显示,在不同的气候变暖情境下,其繁殖领域平均向北平移了3.8到4.4个纬度,这将使迁徙物种例如花园苇莺(Sylvia borin)的迁徙距离增加400到600km,因为越冬栖息地并不会改变。根据计算,这些距离的增加需要鸟类净重量大约增加9%,相当于迁徙过程中所需的能量必须以脂肪的形式储存。我们知道,一只重约15克的小麻雀飞行1000公里需要消耗约3.5克脂肪。这样额外的消耗意味着在移徙中途停留期间要寻找更多的食物,并能够将其作为能量储存在体内,这是一项难以应对的挑战,也是种群减少的原因之一。如果面对新选择压力的遗传适应慢于温度上升的速度,适应迁徙新环境的过程便不够快[13]。

[照片来源:Jean-François Desmet(让-弗朗索瓦·德斯梅特)/保留版权]
由于迁徙行为是由遗传决定且具有遗传特征,这一特征可能会在当前变暖的定向选择压力的影响下进化。一个德国研究团队对于候鸟的实验表明,黑头莺(Sylvia atricapilla)(图8)的迁徙行为可能会由于鸟类回交形成的选择压力而消失或出现。在实验开始时,实验中的部分迁徙种群中有三代完全迁徙,六代完全定居。
在目前的选择压力下,许多部分迁徙物种会变为常驻,正如实验中观察到的,面对迁徙条件不断恶化的新挑战,许多跨撒哈拉沙漠的迁徙物种不得不改变它们的迁徙行为。迁徙行为强度的巨大变化在鸟类历史上屡屡发生,尤其是在冰川和冰川间期交替的更新世期间,鸟类迁徙的速度和方式都发生了显著的变化。
3.3 生境追踪的替代方案:本地适应
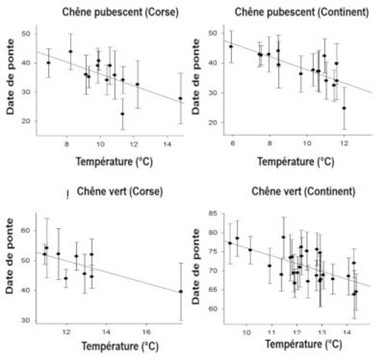
[图片来源:Jacques Blondel(雅克·布隆德尔)]。Chene vert(绿橡树); Corse(科西嘉岛); Date de ponte(产卵日期); Temperature(温度)
气候变暖的不同程度产生了相应的不同生活史特征。例如,鸟类开始在春天更早筑巢[15],但所涉及的机制复杂,因此人们经常观察到例外情况也不足为奇。
生活史性状的变化可能与表型可塑性有关,即个体对环境信号的变化做出及时响应,例如对于当前温度变化,在春天比较冷时,鸟类的产卵时间会迟一些,在温暖时,则早些开始产卵(图9,[16])。
然而这些由于表型可塑性发生的及时响应也存在一定的局限性,因为“表型可塑性窗口”不可伸展,一旦超出其范围,便会发生某种形式的适应不良。维瑟(Visser)等人[17]在一项关于大山雀(parus major)产卵日期变化的研究中惊奇地发现,尽管这些雀形目在繁殖季节取食的落叶橡树毛虫的高峰期提前了大约十天,但它们并未对气候变暖做出响应。
对于这种适应性变化缺乏的解释如下:为了将繁殖时间调整到最合适的时间,以适应所食昆虫种群的峰值丰度,鸟类会利用环境信号(即所谓的近端信号)判断一个月后的食物量(产卵和孵化完成后)。如果决定鸟类产卵的表型可塑性生态因子对温度升高反应不同于鸟类食物的变化,在这个例子中,橡树芽的爆发预示着食叶虫的孵化,在鸟类生殖周期的产卵和育雏两个关键时期便会存在明显差异。鸟类的活动范围很窄,因为当它开始产卵后,一系列现象不可避免地将会紧接着发生:在输卵管中形成卵需要4天,产卵需要1天,后放置在巢穴中孵化12天,而幼鸟在孵化后的11天达到最大,因此,如果一只鸟要产10个蛋,它必须“决定”在食物资源达到峰值之前的4+10+12+11=37天产卵。这需要通过近端信号(在此例中是光周期)来完成此操作[18]。

[照片来源:Jean-François Desmet(让-弗朗索瓦·德斯梅特)/保留版权]
鸟类面临的另一项挑战是需要将需求调整为供应,当供应对应需求的时间发生变化,就会发生失调现象。这种情况并不罕见,特别是在跨撒哈拉的迁徙鸟类当中,数千里以外的物候信号会延迟。如果与它们在欧洲的食物相关的信号已经提前,但是关于返航的信号却没有变化时,就会造成不良适应的结果。科学家在一种生活在荷兰的欧洲斑姬鹟(Ficedula hypoleuca)种群中观察到了这一点,这些鸟从非洲返回的日期并没有因为气候变暖而提前,但是它们用以哺育幼鸟的虫子的出现却因气候变暖而提前了十天[19]。
除了表型可塑性具有一定的限制性以外,通过定向选择的适应性进化可能会很快,以至于选择适应的过程会在几代内影响种群的动态[20]。随着时间的推移,这种进化上的变化将修复同步中断;这一过程被称为“进化拯救”,就是由于定向选择有利于那些最不适的个体,从而引起种群的遗传结构随着时间逐渐变化。

[照片来源:幼虫:太空鸟(CC BY-SA 3.0);成虫:恩托马尔(免版税)]
一种英国煤山雀(图10)提供了一个定向选择的实例[21]。在对该种群有繁殖力的所有个体进行半个世纪的监测后,结果表明从1961到2007年,该山雀的产卵日期提前了14天,进而让该种鸟类能够转而以一种幼虫期的冬蛾(Operophtera brumata)(图11)为食。对每只雌鸟个体的监测发现,每年它们都会调整产卵日期以使其与雏鸟的食物供求相匹配。事实上这种响应是即时的,其食物需求高峰期与毛虫的丰度高峰期之间有显著的相关性,这说明表型可塑性是一种调节机制。
但是表型可塑性的有效性并不是不确定的。有学者认为,当达到表型可塑性的范围极限时,微进化机制便开始起作用。自从所有个体都可以被监测以来,应用于种群的定量遗传学方法已基于两个关键参数证明了该机制的有效性,这两个关键参数是温差造成的选择强度和产卵日期的遗传力。该研究的作者表明,对于在温暖的年份早些筑巢的山雀来说,其幼鸟存活率将增加,而当前一年的气温处于平均水平时,其幼鸟增长率也处于平均水平。
3.4 适应不良的代价

[照片来源:Jean-François Desmet(让-弗朗索瓦·德斯梅特)/保留版权]
对于表型调整以及选择反应的适应性而言,其重要性可以通过估计局部适应不良的代价,例如食物的供求不匹配,从而进行测量。基于蓝雀(图12)繁殖期的最大食物需求量与其幼鸟食用的虫子的丰度值之间的差异,蓝雀模型可被用于测定其适应不良的代价。通过使用双标记水(氧元素和氢元素标记)的方法,可以测量并将结果用能量单位(例如千焦耳)量化,即鸟类为了抚育幼鸟而进行的代谢量。结果表明,与食物供求同步良好的一窝雏鸟相比,食物供求不同步的雏鸟具有以下特征:
- 后代质量低,几乎没有生存的机会;
- 喂养雏鸟的成本更高(雏鸟增重1克需要花费2到4千焦耳,而有良好同步食物供应的只需花费1到2千焦耳);
- 成鸟为喂养食物供求不匹配的雏鸟需要几乎双倍的代谢量(基础代谢的5到7倍,而有良好食物供应的只需基础代谢的3到4倍)[6]。
如果全球变暖加速,而选择性的响应不够快,使生物不能及时适应新环境的变化,这种供应和需求之间的不同步导致的结果将会更加严重。
4. 全球变化与生态系统功能

[图片来源:Jean-François Desmet(让-弗朗索瓦·德斯梅特)/保留版权]
由于群落中所有的物种对气候压力的反应方式各不相同,种间相互作用的破坏可能对生态系统功能产生不可预测的影响。这是一个巨大的问题,因为全球变暖在物种水平方面产生的直接后果是众所周知的,但是我们对种群间因为不断发生重新组合而产生的相互作用几乎一无所知。
这里有一个例子[22]:德国的一种秃鹰,鵟。在过去20年中,其种群数量增加了四倍,其雌鸟的存活率由0.63增加到0.74,雄鸟存活率由0.61增加到0.80。惊奇的是,这些生存变化与同时期的北大西洋气候指标降低有关(NAO,温度越低,降雨量越少,积雪量越大。见《气候变化:北大西洋涛动的例子》)。尽管NAO与该秃鹰的生存之间的关系并没有明确揭示所涉及的机制,因为相关性并不意味着因果关系,但是这些变化在较大的空间范围内得到了证实:当NAO降低,鵟存活率将上升,反之亦然。因此,其包含的机制在一定程度上也与气候因素相关。
这项研究的作者最后发现:该秃鹰的主要猎物,尤其在冬天,是一种对气候极敏感的田鼠。尽管雨水和霜冻对这种小型哺乳动物十分不利,但是如果积雪不太厚,覆盖的雪反而会保护它们不被捕食者发现,也便于捕食者寻找并捕捉猎物。在过去的二十年中,小层积雪的天气时常出现,这既有利于田鼠,也有利于它们的捕食者。秃鹰的生存地理变异和气候条件之间的这种密切关系,说明了不同水平之间的相互依赖性,即气候、田鼠及其捕食者之间的相互依赖性。但是这个例子也说明了气候变化可能让关系进一步复杂化,而这种关系本身也具有出人意料的特点。这使得关于物种集合中食物网的重组,或者生物体对气候变化的响应等研究,将更难进行预测和建模。
5. 补充信息
生物对气候变化的响应研究存在主要限制,这是由于它们之间存在相互关联,而这种关联并没有解释清楚其相关诱因和机制。然而,全球变化中的几个成分所涉及到的分布区和物候的变化,例如鸟类的产卵日期或迁徙出发日期,使得其机理分析变得更为复杂。以下三种方法提供了有趣的回答:
- 首先是对生态位范围的建模[23],该模型基于有机体生态位的多变量统计学,通过将生物的空间分布与关键环境变量相关联,根据IPCC (政府间气候变化专业委员会)提出的不同气候变化预想情景,将所定义的生态位投射到该物种将来可能占据的地理空间中。
- 第二种方法,也是非常有发展前景的一种方法是表观遗传学方法,即通过研究环境起源机制(气候压力、干扰事件、母体效应等),这些机制可以调节编码基因的表达而不改变生物体的遗传结构(参见《表观遗传学:基因组及其环境》)。这些非孟德尔遗传(也被称为“软遗传”)在生物适应环境变化方面发挥着重要的作用,因为他们在个体发育中表达,不需要经过自然选择的代际筛选。此外,这些表观遗传起源的变化是可遗传的,其进化结果是将一系列均值转化为最优值,或者改变其方差,或者减小新的最优值附近的方差,这被称为“表型通道化”过程[24]。
- 第三种方法是使用过去的数据,虽尚未得到充分利用,但很有前景。我们一旦能识别到生物对过去变化产生响应,便可以开展“自然实验”, 从而验证生态学和进化论的相关原理[25]。
因此,化石记录可以在几年到几万年的时间尺度上,提供响应气候变化的适应性进化的数据。它也可以提供在几年到几千年的较短时间尺度上关于表型可塑性真实性的数据。古基因组学可以识别范围变化、种群衰退和灭绝事件等对气候变化的相关响应。因此,科学家已使用进化遗传学和“复活生态学”的实验方法探索了对气候变化的响应,其中包括使繁殖体处于长时间休眠状态,有时长达几个世纪(例如硅藻,昆虫,真菌藻类,细菌),然后进行实验,将它们暴露在气候变化胁迫事件中[26]。所有这些方法的优点(其中许多方法仍处于探索阶段),是它们能够让我们的研究模型从相关模型过渡到可以识别所涉及机制的过程模型。
6. 要记住的信息
- 生物在其温度耐受范围之间生存,低于或超出该范围都会影响其生存,
- 生境追踪是对气候变暖一种经典响应,因物种而异,
- 在极端生境(苔原,高山)中不能进行生境追踪,因此鸟类身处这些极端环境中会对其生存造成威胁,
- 全球变暖对候鸟,特别是跨撒哈拉沙漠迁徙的鸟类,施加了新的严峻的限制,
- 生境追踪的一种替代方法是通过表型调整和/或微观进化来进行局部适应,
- 全球变化改变了物种之间的相互作用,从而改变了群落的结构和动态。
参考资料与说明
封面插图。 [来源:Jacques Blondel(雅克·布隆德尔)]
[1] 事实证明,人类活动导致的地球系统的变化是深刻且普遍的,因此气象学家和化学家保罗·约瑟夫·克鲁岑(Paul Josef Crutzen)在20世纪末创造了“人类世”一词(克鲁岑&斯托尔默(Crutzen & Stoermer), 2000, “人类世”, 全球变化通讯. 41, 12-13),从字面意思理解,“人类世”指的是地球历史上人类活动在地壳和强大地磁力留下不可逆转标记的时期。尽管国际地层学委员会对地球历史的新序列持谨慎态度,但这个名词很快成为科学词汇的一部分,也被纳入了生命科学词汇。
[2] Parmesan, C. 2006. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. System 37: 637-669.
[3] Pearce-Higgins, J. W. & Green, R. 2014. Birds and climate change. Impacts and conservation responses. Cambridge, Cambridge Univ. Press.
[4] Sekercioglu, C. H., Schneider, S. H., S. H., Fay, J. P. & Loarie, S. R. 2008. Climate change, elevational range shifts, and bird extinctions. Cons. Biol. 22: 140-150.
[5] Brown, J. H., Gillooly, J. F., Allen, A. P., Savage, V. M. & West, G. B. 2004. Toward a metabolic theory of ecology. Ecology 85: 1771-1789.
[6] Hewitt, G. M. 2004. Genetic consequences of climatic oscillations in the Quaternary. Phil. Trans. R. Soc Lond. B 359: 183-195.
[7] Devictor, V., Julliard, R., Couvet, D. & Jiguet, F. 2008. Birds are tracking climate warming but not fast enough. Proc. R. Soc. Lond. B 275: 2743-2748.
[8] Devictor, V. van Swaay, C. Brereton, T. Brotons, L. Chamberlain, D. Heliölä, J. Herrando, S. Julliard, R. Kuussaari, M. Lindström, Å. Reif, J. Roy, D. B. Schweiger, O. Settele, J. Stefanescu, C. Van Strien, A. Van Turnhout, C. Vermouzek, Z. WallisDeVries, M. Wynhoff, I. & Jiguet, F. (2012) Differences in the climate debts of birds and butterflies at a continental scale. Nature Climate Change. 2, 121–124.
[9] Burton, J. F. 1995. Birds and Climate Change. London, Christopher Helm.
[10] Devictor, V., Julliard, R., Couvet, D. and Jiguet, F. (2007) French birds lag behind climate warming. Nature Proceedings 10 p. DOI: 10.1038/npre.2007.1275.
[11] Piersma, T. 2007. Using the power of comparison to explain habitat use and migration strategies of shorebirds worldwide. J. Ornithol. 148: S45-S59.
[12] Berthold, P., Fiedler, W., Schlenker, R. & Querner, U. 1998. 25-year study of the population development of Central European songbirds: a general decline, most evident in long-distance migrants. Die Naturwissenschaften. 85: 350-353.
[13] Doswald, N., Willis, S. G., Collingham, Y. C., Pain, D. J., Green, R. E. & Huntley, B. 2009. Potential impacts of climate change on the breeding and non-breeding ranges and migration distance of European Sylvia warblers. J. Biogeogr. 36: 1194-1208.
[14] Berthold, P. 1991. Genetic control of migratory behaviour in birds. Trends Ecol. Evol. 6: 254-257.
[15] Crick, H. Q. P., Dudley, C., Glue, D. E. & Thomson, D. L. 1997. UK birds are laying eggs earlier. Nature 338: 526.
[16] Thomas D. W., Blondel J., Perret P., Lambrechts M. M. & Speakman J. R. 2001. Energetic and fitness costs of mismatching resource supply and demand in seasonally breeding birds. Science 291: 2598-2600.
[17] Visser, M. E., Adriaensen, F., van Balen, J. H., Blondel, J., Dhondt, A. A., van Dongen, S., du Feu, C., Ivankina, E. V., Kerimov, A. B., de Laet, J., Matthysen, E., McCleery, R., Orell, M. & Thomson, D. L. 2003. Variable responses to large-scale climate change in European Parus populations. Proceedings of the Royal Society of London, series B 270: 367-372.
[18] Lambrechts, M. M., Blondel, J., Maistre, M. & Perret, P. 1997. A single response mechanism is responsible for evolutionary adaptive variation in a bird’s laying date. Proc. Natl. Acad. Sci. USA. 94: 5153-5155.
[19] Both, C. & Visser, M. E. 2001. Adjustment to climate change is constrained by arrival date in a long distance migrant bird. Nature 411: 296-298.
[20] Gingerich, P.D. 2009. Rates of evolution. Annu. Rev. Ecol. Evol. System 40: 657-75.
[21] Charmantier, A., McCleery, R. H., Cole, L. R., Perrins, C. M., Kruuk, L. E. B. & Sheldon, B. C. 2008. Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science 320: 800-803.
[22] Jonker, R. M., Chakarov, N. & Krüger, O. 2014. Climate change and habitat heterogeneity drive a population increase in Common Buzzards Buteo buteo through effects on survival. Ibis 156: 97-106.
[23] Lavergne, S., Mouquet, S., Thuiller, W. & Ronce, O. 2010. Biodiversity and climate change: Integrating evolutionary and ecological responses of species and communities. Annu. Rev. Ecol. Evol. System 41: 321-350.
[24] Rey, O., Danchin, E., Mirouze, M., Lootù, C. & Blanchet, S. 2016. Adaptation to global change: a Transposable Element-Epigenetics perspective. Trends Ecol. Evol. 31: 514-526.
[25] Nogues-Bravo, D., Rodriguez-Sanchez, F., Orsini, L., de Boer, E., Jansson, R., Morlon, H., Fordham, D. A. & Jackson, S. T. 2018. Cracking the code of biodiversity responses to past climate change. Trends Ecol. Evol. 33: 765-767.
[26] Orsini, L., Schwenk, K., De Meester, L., Colbourne, J.K., Pfrender, M.E. & Weider, L.J. 2013. The evolutionary time machine: using dormant propagules to forecast how populations can adapt to changing environments. Trends Ecol. Evol. 28: 274-282.
环境百科全书由环境和能源百科全书协会出版 (www.a3e.fr),该协会与格勒诺布尔阿尔卑斯大学和格勒诺布尔INP有合同关系,并由法国科学院赞助。
引用这篇文章: BLONDEL Jacques (2024年2月23日), 鸟类如何适应气候变化?, 环境百科全书,咨询于 2025年4月24日 [在线ISSN 2555-0950]网址: https://www.encyclopedie-environnement.org/zh/vivant-zh/how-birds-adapt-changing-climate/.
环境百科全书中的文章是根据知识共享BY-NC-SA许可条款提供的,该许可授权复制的条件是:引用来源,不作商业使用,共享相同的初始条件,并且在每次重复使用或分发时复制知识共享BY-NC-SA许可声明。









