极端环境微生物

地球上鲜有不毛之地。冰川沙漠、温泉、海底、高盐环境、地幔的岩石……即使在这些恶劣的环境下,一些微生物依然能够构成丰富的生物多样性。这些生物被称为极端环境微生物(extremophilic microbes),对它们的研究揭示了不同于细菌和真核生物的第三种生命形式——古菌(Archaea)。这类有机体大量存在于环境中,与我们的细胞存在进化上的关联,其表现出的独特模式有助于理解复杂细胞过程的起源与运作。在任何其他生命形式都无法存活的温度、压力或盐度条件下,极端微生物采取不同策略,来维持自身细胞结构的完整性。对它们的研究揭示了生物在“艰苦的”生态系统中开疆扩土的能力。目前,人类正在利用这类微生物酶的特殊属性,开发清洁可持续的工业流程。
1. 极端微生物的生物多样性
土壤与海洋微生物支配着我们的星球[1]。它们在调节主要的地球化学循环方面扮演着重要角色,并有望为未来技术提供大量新型生物催化剂。但由于当前的微生物学仍过度局限于人类健康问题,我们对这类微生物多样性的了解仍然非常片面。只有不到1%的环境微生物能在实验室中进行纯培养。过去十年间,新工具(如可以直接分析微生物群落DNA的宏基因组学)的发展改变了我们对微生物生态系统的看法。【生态系统:一系列相关联的生物(又称生物群落)及其所处环境(又称群落生境)构成的统一整体,涉及生物、地理、土壤、水文、气候等多个层面。生态系统的特征包括物种与物种间、物种与周围环境间的相互作用,生态系统各组分间赖以维持生命的物质和能量流动,以及久而久之建立起的稳态与进化间的动态平衡。】[2]得益于上述新技术,我们发现,许多长久以来因其极端的物理化学条件而被认为不适宜生物存活的环境实际上具有丰富的微生物生命形式[3]。

[照片来源:© Ifremer,法国海洋开发研究所]
例如,火山热液泉就有着惊人的生物多样性,可谓深渊尽头的生命绿洲(图1)(详见焦点:《黑烟下的生态系统》);大型盐湖或死海等高盐环境也是某些微生物的栖息地,这些微生物只有在其他任何生命形式都无法承受的超高盐度中才能生长;冰川和极地环境同样孕育有大量微生物;此外,在海底、沉积物和深部地层中也存在着极丰富的微生物群落。据估计,80%的陆地生态系统常年暴露在5℃以下的低温中,且经常处于高压条件下。因此,放眼全球,极端微生物的存在不应再被视为个例。
人们对极端微生物在生态系统中的作用(包括气候调节)知之甚少。最近的研究表明,它们在温室气体的产生,以及主要的碳、氮和硝酸盐循环方面都发挥着不容忽视的作用。通常来说,在这类微生物的基因组中,90%以上的基因所编码的蛋白质都有着不为人知的功能。之所以会出现这种情况,是因为生物大分子必须适应上述极端环境特有的物理-化学环境、营养和能源。这些环境限制可能催生新的代谢途径,其使用的底物和辅助因子与“常规”生物体所使用的不同。
2. 古菌的发现:革命性事件
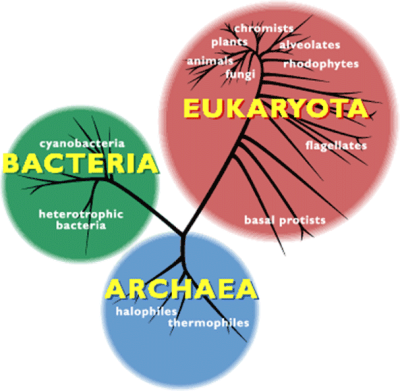
BACTERIA细菌,cyanobacteria蓝细菌,heterotrophic bacteria异养细菌,EUKARYOTA真核生物,fungi真菌,animals动物,plants植物,chromists茸鞭生物,alveolates囊泡虫,rhodophytes红藻,flagellates鞭毛虫,basal protists基础原生生物,ARCHAEA古菌,halophiles嗜盐菌,thermophiles嗜热菌
许多极端环境有机物都是古菌。作为独立于细菌和真核生物之外的第三种生命形式(图2),这类微生物有着明显不同于二者的分子特征。1990年,美国生物学家卡尔·乌斯(Karl Woese)首次提出了“古菌”这一定义。乌斯尝试以核糖体RNA为分子示踪剂,重建整体的生命进化树。自那之后,越来越多的新型古菌便不断被发现,对其基因组的研究也证实,它们的确属于一种未知的全新生命形式[4]。同细菌一样,古菌也是没有细胞核的单细胞生物(图3),但它们却有着独树一帜的鲜明特征。这些微生物不仅有自身专属的病毒,更令人惊讶的是,有些古菌还能够与海绵等复杂有机体形成共生关系。【共生:两个分属不同物种的有机体之间建立的亲密、持久、互利的联系。参与共生的有机体称为共生体,其中,较大的共生体也可称为宿主。】古菌在肠道菌群中也有发现,它们对人类健康非常重要(详见《人类微生物:健康的盟友》)。目前,在古菌中尚未发现病原体,但其已被证实与特定疾病或代谢紊乱(如肥胖等)有关。古菌有着同细菌一样复杂的进化与扩张过程,且与真核生物的进化关系惊人的接近。有些假说认为,古菌在进化树上位于真核生物起源处[5](详见《共生与进化》)。
[显微照片来源:© Ifremer]
古菌似乎格外适合在地球上已知最极端的环境中生长。因此,人们最初认为它们多样性极低,只存在于火山热液和高盐湖泊处。这一早期论断完全有失偏颇。分子生态学研究表明,所有地球环境中都存在大量古菌系。事实上,我们正在发现越来越多的“非极端环境”古菌,或将占海洋和土壤微生物总量的25%[3]。因此,古菌不应再被视作一种边缘、古老或原始的生命形式。
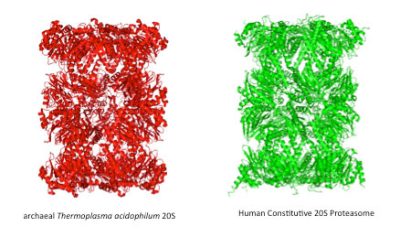
古菌界的这一发现成果意义重大,但其重要性仍远未得到充分认识。这一发现改变了我们将所有生物划分为原核和真核生物两类的观点。对古菌进化过程的研究开拓了解释“细胞生命如何产生”这一问题的新思路[6]。尽管古菌是没有细胞核的单细胞生物,但它们与包括人类细胞在内的真核细胞有许多相同的生命过程[7]。越来越多的证据表明,古菌适合成为分子生物学的模式生物。事实上,一直以来,研究人类细胞中的大型细胞器都非常困难。在实验室条件下,很难控制细胞器的纯化及其活动。所幸,在古菌中发现的同源系统提供了上述细胞器的简易版,不仅比原版稳定得多,而且更加易得。此外,极端生长环境赋予古菌的特性也使其能够更好地被激活或抑制。这些优势加上近年来基因工具的发展,使得古菌成为研究整合生物学(融合在体研究、生物化学研究、生物物理学研究和结构生物学研究的综合性学科)的极佳模式生物。这些研究首次确定了许多细胞器的结构,为包括抗肿瘤药物在内的多种药物研发提供了来源(图4)。
3. 极端环境微生物与生命的极限
生物体的生长发育需要:
- 碳源,构成生物大分子的基本元素;
- 水,蛋白质行使功能最常用的溶剂;
- 能量,生物系统行使功能的必要条件。
能量由光、电子供体元素(如金属)、酶催化下的化学键断裂等途径提供。在一直被认为是不毛之地的环境中发现了微生物群落,这一事实表明,生物生存的物理化学极限远比人们从前认为的要大得多。这些统称为极端微生物的有机体并没有处于“挣扎求生”的状态,而是真的需要那些在人类看来极度艰苦的环境,也只能生长于这些环境[8]。
在热液泉(详见焦点:《黑烟下的生态系统》)中,存在名为“嗜热(thermophilic)菌”或“超级嗜热(hyperthermophilic)菌”的古菌和细菌,它们只有在高温下才能达到最好的生长状态,有时环境温度甚至需要超过110℃。海底(如马里亚纳海沟)独有的某些嗜压菌(piezophiles)则需要在20MPa以上的压力环境中才能生存[9]。在海底、湖泊和冰川环境中,“嗜冷微生物”(psychophiles)只能活跃于15℃以下,甚至低至-12℃的环境中[10]。在高盐环境中,自由水大多结合盐离子,因而十分稀少。这样的环境会破坏细胞,导致蛋白质变性。然而,被称为“嗜盐”(halophilic)有机体的生物具有独特的生物化学特性,可以在严重脱水状态下保持细胞的完整性[11]。热液泉、地层流体或高盐环境通常呈现出极端的pH值(0和13)。因此,绝大多数在此生存的有机体具有多种嗜性。
除了极端且经常大幅波动的物理-化学环境,极端微生物还面临多种环境压力,如辐射或重金属。这些因素能够产生自由基,破坏DNA和蛋白质。绝大多数极端微生物都发育出了DNA修复系统和蛋白质循环系统,帮助自身抵抗高达10000格雷的辐射。极端微生物研究的另一个前沿领域在于,极端环境微生物似乎特别适应极低能量和(或)营养流动导致的生存压力,部分极端微生物甚至可以在海底沉积的泥沙深处或地下数千公里的地幔内部缓慢地存活和生长[13]。因此,对极端微生物的研究还可以促进环境能源的开发。
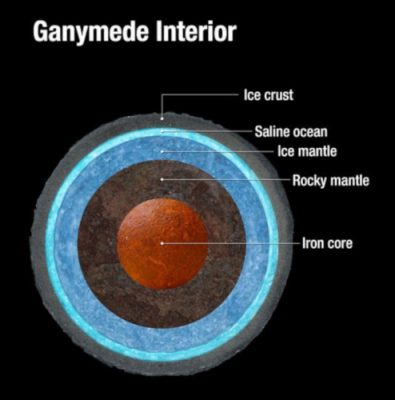
[图源:Felicia Chou,美国国家航空航天局]
Ganymede Interior木卫三内部,Ice crust冰地壳,Saline ocean盐海,Ice mantle冰地幔,Rocky mantle岩石地幔,Iron core铁地核
对古菌,或更广义范畴上的极端微生物的研究和发现,改变了我们对地球宜居性的观念。这些生物的存在为我们在其他星球上寻找可能的生命痕迹指明了方向[13]。例如,在智利的阿塔卡马沙漠,人们发现了能存活于干旱和寒冷环境中的微生物。嗜盐有机体可以在-15℃的环境中生长。此外,在发现火星上的液态盐池、土卫和木卫(如木卫二和木卫三)上的深海和热液活动后(图5),与高压环境密切相关的微生物也是值得进一步思考的有趣观察。
4. 极端微生物如何维持生物功能?
维持生物膜的稳定和正常功能,是细胞在极端温度、压力或盐度条件下生存的首要条件。膜结构对产生能量和分隔生化反应至关重要。膜脂质组分的变化能够帮助其适应影响膜流动性的高(低)温和高压。
对非极端环境有机体而言,暴露于“极端”物理-化学环境会使某些蛋白质失活甚至变性。生物体对极端环境的适应性还表现为维持细胞器的合成,以及维持组成蛋白质的多肽链的三维折叠,这种折叠能够赋予酶生化活性。理解极端环境下生物大分子的稳定机制,有助于理解诸多内容,包括但不限于以下三点:
(i)生物的起源和扩张能力;
(ii)调控蛋白质行使功能的基本过程;
(iii)维持细胞器完整性的广谱细胞机制[8]。
在维持细胞组分方面,极端微生物广泛使用的首要策略是在细胞质中合成并积累海藻糖、甜菜碱等小分子,从而稳定分子结构。维持蛋白质完整性和稳态还需要优化分子伴侣和蛋白质修饰系统,以防止蛋白质聚集,协助多肽链折叠,或者激活细胞内的蛋白酶,促使其启动快速降解过程。蛋白质的这些“质量控制”系统对于嗜热和嗜冷微生物的适应力都至关重要。这类系统并非极端微生物所特有,而是存留在包括人类在内的所有生命体当中。因此,极端环境微生物系统可作为一种简化模型,帮助理解应激反应、退行性疾病和衰老过程等基本机制。
维持生物大分子完整性的细胞机制需要细胞提供大量能量,这是因为真正的极端微生物蛋白质都是高度修饰的。这些修饰在进化中经突变获得,有助于生物体在高温、高盐或高压条件下维持自身蛋白质的稳定性。然而,对比极端环境有机体与相应的常温环境有机体的蛋白质晶体结构发现,二者的整体结构几乎没有差别。另一方面,选择性突变产生了极大的生物物理特性差异。例如,嗜热蛋白质在室温下即被“冻结”,其原因在于,分子内相互作用的优化增强了大分子的结构,从而赋予了蛋白质非凡的稳定性。然而,尽管维持三维结构对生物大分子正常行使功能至关重要,但是蛋白质还需有整体的动态性。有些区域必须通过移动来识别底物和辅因子,执行复杂的生物化学功能。
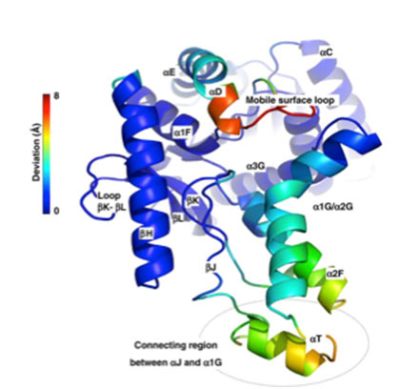
[来源:Coquelle等,2010,详见参考资料及说明[14]](Loop环,Deviation偏离角,Mobile surface loop可动表面环,Connecting region between αJ and α1GαJ和α1G间的连接区))
多亏了与分子进化相关的结构比较分析及模拟工作,蛋白质结构内的关键区域现已确定(图6)[14]。在进化过程中,这些区域的氨基酸被取代。整体稳定性和局部动态性之间的最佳平衡使得蛋白质能够在极端环境中维持其功能。分子折叠的类型多样,因此蛋白质结构适应环境限制的策略也各不相同。除了有助于理解极端微生物,这一工作也增进了人们对生物大分子生成以及酶的作用机制的理解。当面向具有临床价值的蛋白质时,上述研究可用于药物开发。
不同环境条件对分子结构的限制不同,相应的适应性策略也不同。因此,对于嗜热菌来说,主要的挑战在于蛋白质在高温下难以折叠,在这种情况下,适应性策略表现为强化相关的力,帮助蛋白质稳定折叠,同时在酶行使生化功能的区域维持一定的灵活性。低温的主要影响在于减慢化学反应速率。在嗜冷菌的蛋白质中,适应性策略表现为修饰活性位点,继而提高催化效率,该策略关乎分子内部整体或局部限制的放松[10]。这些修饰将代谢维持在较低但足以进行细胞分裂的水平。盐既能减少自由水的数量,又能与多肽链相互作用,这些效应将影响蛋白质的溶解度,破坏蛋白质折叠所需的分子内界面。然而,嗜盐菌在进化中积累了许多有利突变,这些突变能够产生帮助抵消这些效应的蛋白质,甚至化盐离子的危害为优势[15]。多种突变叠加,有助于稳定结构,同时维持系统正常运作所必需的水化层。上述适应性策略非常先进,以至于这些有机体的绝大多数蛋白质已经发展到只在高盐环境下溶解和折叠[16]。最后,近期研究表明,针对海底和地球地质层深处的高静水压力,极端微生物也进化出了相应的适应性策略[9]。在这种情况下,修饰的主要对象为存在于分子结构中的空洞。
在与“极端”环境生命相关的各种适应性策略中,蛋白质结构的变化深刻地改变了生物系统的生化和生理过程。因此,我们眼中的“正常”环境:温度37℃、盐浓度3%、大气压、有氧等,对绝大多数极端微生物而言其实是很严峻的,会对细胞造成压力。例如,水是嗜盐菌的致命溶剂[17]。因为这类有机体为了保证其蛋白质的溶解性与正确折叠,在细胞质中积累了大量的盐类,浓度接近饱和。在地质尺度上,地球表面不同区域的气候大相径庭,形成的“极端环境”也差别巨大。也正因如此,“极端微生物”实际上是一个相对的概念。
5. “极端酶”在生物技术中的作用
[图源:© Ifremer]
酶作为一种天然产物,能够高效、无污染地进行化学反应。在粮食和环境危机的大背景下,人类需要发展仿生经济,正因如此,在各类极端环境微生物群体基因组中发现的新酶,或称极端酶(extremozymes)引起了广泛的研究兴趣[18]。的确,这些酶效果强劲,能够在极端条件下发挥作用,有时还可以催化独特的化学反应,这些有趣的特质赋予了它们多种多样的应用,例如,在生物科技领域,利用极端酶生产生物燃料、生物材料或药物分子。嗜盐微生物的酶能在高盐环境、有机溶剂和较广的pH条件下发挥作用,可用于食品加工、造纸业和纺织业。
来自嗜热和(或)嗜压有机体的嗜热酶和嗜压酶极其稳定,可用于在无菌条件下加工食品。它们能够适应多种物理-化学条件,也可用于纺织、皮革、化妆品或制药等多种工业流程。由于其性质独特、数量众多,涉及大量相互作用和共生关系,因而能够在极端环境中调控细菌和古菌群落的动态性,这类微生物几乎相当于一座未经开发的基因资源宝库(图7)。因此,基于极端环境微生物多样性的新型生物催化剂和抗生素研究已成为一个迅速发展的学科,亟待开发专用的酶学和结构学筛选和鉴定平台。
参考资料及说明
封面照片: [来源:© Bruno Franzetti]
[1] Abdoun E. (2014) Science & Vie 1161, 70-77
[2] Banik J.J. & Brady S.F. (2010) Current opinion in microbiology 13, 603-609
[3] Cowan D.A., Ramond J.B., Makhalanyane T.P. & De Maayer P. (2015) Curr Opin Microbiol 25, 97-102
[4] Gribaldo S., Forterre P. & Brochier-Armanet C. (2011) Research in microbiology 162, 1-4
[5] Forterre P. (2015) Frontiers in microbiology 6, 717
[6] Gribaldo S, Forterre P, Brochier-Armanet C. (2008) Les ARCHAEA : Evolution et diversité du troisième domaine du vivant, Bull. Soc. Fr. Microbiol. 23(3):137-145
[7] Brochier-Armanet C., Forterre P. & Gribaldo S. (2011) Curr Opin Microbiol 14, 274-281
[8] Oger P. & Franzetti B. (2012) Biofutur 336, 36-39
[9] Jebbar M., Franzetti B., Girard E. & Oger P. (2015) Extremophiles 19, 721-740
[10] Cavicchioli R., Siddiqui K.S., Andrews D. & Sowers K.R. (2002) Current Opin Biotech 13, 253-261
[11] Oren A. (2015) Current Opin Biotech 33, 119-124
[12] Inagaki F. et al (2015) Science 349, 420-424
[13] McKay C.P. (2014) Proc Natl Acad Sci USA 111, 12628-12633
[14] Coquelle N., Fioravanti E., Weik M., Vellieux F., & Madern D. (2007) J Mol Biol 374, 547-562
[15] Madern D., Ebel C. & Zaccai G. (2000) Extremophiles 4, 91-98
[16] Vauclare P., Marty V., Fabiani E., Martinez N., Jasnin M., Gabel F., Peters J., Zaccai G. & Franzetti B. (2015) Extremophiles 19, 1099-1107
[17] Franzetti B. (2010) Biofutur, 35-38
[18] Raddadi N., Cherif A., Daffonchio D., Neifar M. & Fava F. (2015) Applied microbiology and biotechnology 99, 7907-7913
环境百科全书由环境和能源百科全书协会出版 (www.a3e.fr),该协会与格勒诺布尔阿尔卑斯大学和格勒诺布尔INP有合同关系,并由法国科学院赞助。
引用这篇文章: FRANZETTI Bruno (2024年3月14日), 极端环境微生物, 环境百科全书,咨询于 2025年1月21日 [在线ISSN 2555-0950]网址: https://www.encyclopedie-environnement.org/zh/vivant-zh/microbes-in-extreme-environments/.
环境百科全书中的文章是根据知识共享BY-NC-SA许可条款提供的,该许可授权复制的条件是:引用来源,不作商业使用,共享相同的初始条件,并且在每次重复使用或分发时复制知识共享BY-NC-SA许可声明。









