Nitrates in the environment
PDF
The agricultural origin of nitrate leaks into the environment is no longer disputed. It results from the natural functioning of the nitrogen cycle and the use of fertilizing materials. The presence of low-concentration nitrates in the environment is a phenomenon that is difficult to circumvent. However, their concentrations in the natural environment and in the products we consume are highly dependent on the agricultural practices implemented. This article attempts to shed light on the questions raised by nitrogen management in agricultural soils, which is the source of these nitrates. It concludes that nitrogen fertilization management requires the development of new strategies that should be helped to emerge.
- 1. Intensification of agriculture and nitrate leakage into the environment
- 2. Mechanisms of nitrate formation in soil and water
- 3. Is increasing the nitrate content of water dangerous to health?
- 4. Is increasing the nitrate content of water or soil detrimental to the environment?
- 5. Can agriculture reduce the nitrogen leakage it causes into the environment?
1. Intensification of agriculture and nitrate leakage into the environment
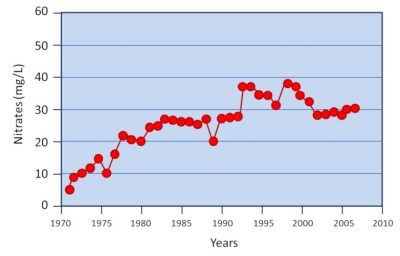
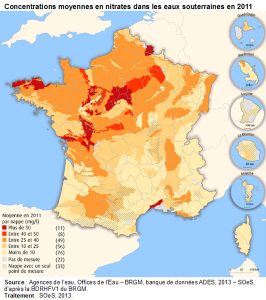
2. Mechanisms of nitrate formation in soil and water
The formation of nitrates in soil and water is a stage in the nitrogen cycle that functions naturally in the environment (Figure 3) under the action of more or less specific microflora, present in soil and water.
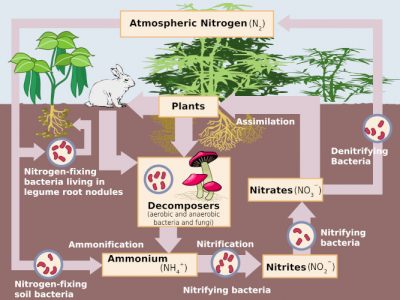
In an oxygen-deficient environment, these nitrates can also be used by certain soil bacteria for respiration in place of oxygen: they undergo denitrification, which transforms these nitrates into nitrites and then into gaseous nitrogen oxides (NO and N2O) and finally into inert atmospheric nitrogen (N2), thus returning to the starting point of the cycle [3].
The presence of nitrates in the soil is therefore a result of all these transformations. In natural environments, the nitrate concentration in soil or water is generally low, indicating that the nitric nitrogen formed is quickly taken up by plant assimilation or microbial transformations. The availability of usable nitrogen thus often appears to be a limiting factor in these transformations. However, nitrate concentrations may be higher in media enriched with organic matter, either naturally or under human action. Thus the accumulations of guanoProduct resulting from the accumulation and evolution of landbird and marine bird droppings in the coastal territories of South America. Very rich in organic and nitric nitrogen it gave rise to a large farm in Europe and the United States in the 19th century. in Chile were a source of natural nitrates used in agriculture, while the natural formations of saltpeter on our limestone walls are the result of domestic nitrogen emissions in the vicinity of homes.
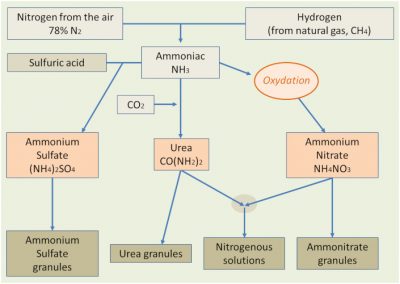
The industrial manufacture of nitrogenous fertilizers (Figure 4), mainly in the form of ammonia, urea or nitric products, has had an undeniable effect on the increase in crop production, with an increase in crop yields in direct response to the supply of these fertilizers. But these inputs of fertilizing elements have also resulted in an amplification of nitrogen transformations in the natural environment, which has resulted in an increase in the above-mentioned leaks.
3. Is increasing the nitrate content of water dangerous to health?
This issue has generated much debate within the human health protection community. The high solubility of nitrates promotes their diffusion in the soil and facilitates their assimilation by plants. But it is also the cause of their escape from root zones in periods of heavy rainfall, and their migration to groundwater or rivers. Our living environment is therefore a natural environment in which we live in the presence of nitrates. In our diet we regularly consume nitrates, usually in low doses. They are provided by vegetables and fruits, by water that has filtered through soils more or less enriched in organic matter, or even by products enriched with nitrates or nitrites used as food preservatives. The increase in nitrate levels in river and groundwater over the past century has raised questions about their toxicity. Synthesis work has thus led the World Health Organization (WHO) [4] to define an acceptable daily intake (ADI) of nitrates in the diet according to the weight of the person: 3.7 mg NO3–/kg/day, generally reduced to 250 mg/day for a person weighing 70 kg.
This absorption of nitrates, at low doses, is considered by some to be beneficial to health insofar as it has been shown to have positive effects on blood pressure and cardiac function [5]. However, the attention of the health authorities has focused mainly on two identified risks following such absorption: blue infant disease or methemoglobinemia, and the possibility of production of carcinogenic nitrosamines from nitrites derived from nitrates. We know that nitrates can be transformed naturally into nitrites by the microorganisms in our digestive tract. These nitrites, more reactive compounds than nitrates, can pass through the blood and bind to hemoglobin, inhibiting its functioning for oxygen transport when formed in large quantities.
Infant methemoglobinemia is thus attributed to this formation of nitrites due to excessive absorption of nitrates by water or by food products that are too rich in nitrates. Epidemiological observations [6] led the WHO to define a standard for the maximum acceptable concentration of nitrates in drinking water (50 mg/l) mainly based on the risk of this pathology: although discussed, the relevance of this standard is reaffirmed by the WHO [5] which also highlights the increased risks for young children in the event of gastrointestinal infection, conditions favourable to the reduction of nitrates to nitrite.
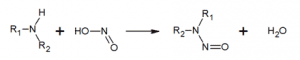
In conclusion, the consumption of low-dose nitrates in our diet and in the water we drink is almost inevitable, and is considered to have no harmful effects on our health. Consumption at higher doses is not without risk: this is the reason for the permanent monitoring of nitrate levels in water distribution networks recommended by the WHO.
4. Is increasing the nitrate content of water or soil detrimental to the environment?
4.1. Nitrates and eutrophication of aquatic environments
Surface waters (rivers and lacustrine environments) and waters of marine coasts can be enriched by leaks of mineral nitrogenCombined forms of nitrogen with oxygen or hydrogen, namely nitrates (NO3-) ; nitrites (NO2–) and ammonia salts (NH4+). emissions, mainly nitrates, from cultivated or urbanized areas. These leaks are the result of excessive inputs of fertilizers or nitrogenous organic products, and their transport to watercourses or surface reservoirs. These waters then become favourable environments for the development of aquatic plants and algae, which leads to eutrophication.
The supply of nitrogenous nutrients, often accompanied by phosphorus in the discharge of domestic wastewater into the river or leaks of animal effluents (manure, slurry) spread on the fields, allows the proliferation of plant biomass in the water. When it dies, its decomposition leads to oxygen consumption at the expense of aquatic fauna and creates putrefaction conditions with the production of malodorous compounds, particularly hydrogen sulphide (H2S). In France, these eutrophication phenomena have been observed in particular in the Alpine lakes, where they have been controlled (Lake Annecy) or have declined significantly as a result of the reduction in phosphate pollution (Lake Geneva, Lake Bourget [10]).
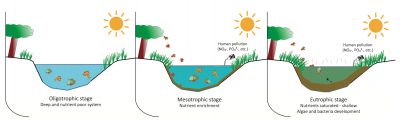

On the other hand, in coastal environments or surface reservoirs (Figure 7) where significant amounts of phosphorus have been accumulated as a result of overloading of livestock manure and its entrainment by surface runoff from the soil, the availability of phosphates is not a limiting factor in algal development. The main way to limit their proliferation observed in spring is to reduce the availability of nitrogen, mainly in the form of nitrates [1].
4.2. Soil nitrates and nitrogen oxide emissions
When in an oxygen-deficient environment, nitrates can be used in the respiratory processes of denitrification bacteria, which will successively lead to less oxidized nitrogen compounds depending on the sequence of reactions:
Nitrates (NO3–) → Nitrites (NO2–) → Nitric oxide (NO) → Nitrous oxide (N2O) → Gaseous nitrogen (N2)
During these transformations, nitric oxide (NO) and nitrous oxide or nitrous oxide or nitrous oxide (N2O) may be emitted from the environments where these transformations occur, including the soil. These two gases are air pollutants. Nitric oxide is a precursor of ozone that is harmful to vegetation in the lower layers of the atmosphere. However, these nitric oxide emissions from soil nitrates are of low importance compared to other anthropogenic sources, particularly those from vehicles. On the other hand, nitrous oxide is a powerful greenhouse gas: 1000 times less concentrated than carbon dioxide, it has a global warming potentialMeasurement unit, expressed in CO2 equivalent, allowing the warming capacities of the various greenhouse gases to be compared on the basis of their radiative properties and lifetime. 300 times stronger, and contributes 7.5% to the increase in the greenhouse effect [11]. Its emissions during denitrification are the main natural source of this gas, the increase in concentration in the atmosphere of which is directly related to the development of nitrogen fertilizers. Limiting these emissions is one of the current challenges in nitrogen management in agriculture, in particular by seeking to limit the availability of nitrates during periods when plants do not need them.
4.3. Nitrates and the destruction of soil organic matter
Nitrates, the most oxidized form of nitrogen, can be assimilated by plants and are then used to make organic compounds. But if they are not used, these nitrates are eventually denitrified. The overall balance of the nitrogen cycle suggests that combined nitrogen inputs in the form of nitrogen fertilizer return to the atmosphere in the more or less long term through this denitrification. However, this reaction is coupled with an oxidation of organic matter (CH2O), according to the equation :
4 NO3– + 4 H+ + 5 CH2O → 5 CO2 + 2 N2 + 7 H2O
Agronomists know that agricultural practices stimulate the mineralization of soil organic matter, highlighting in particular the favourable effect of tillage on soil aeration and the oxidative activity of microflora. However, it is important to bear in mind that the contribution of 100 kg/ha of nitrogen in nitric form constitutes a capacity for oxidation (and therefore elimination) of an equivalent quantity (107 kg/ha) of organic carbon from the soil by this denitrification mechanism. Such an amount is not negligible in terms of managing soil organic matter, which is also being preserved for its beneficial effects on the physical and biological functioning of the soil. This mechanism for destroying organic matter is not taken into account in the analysis of the evolution of the soil’s carbon stock and in the reflection on its management to preserve it.
5. Can agriculture reduce the nitrogen leakage it causes into the environment?
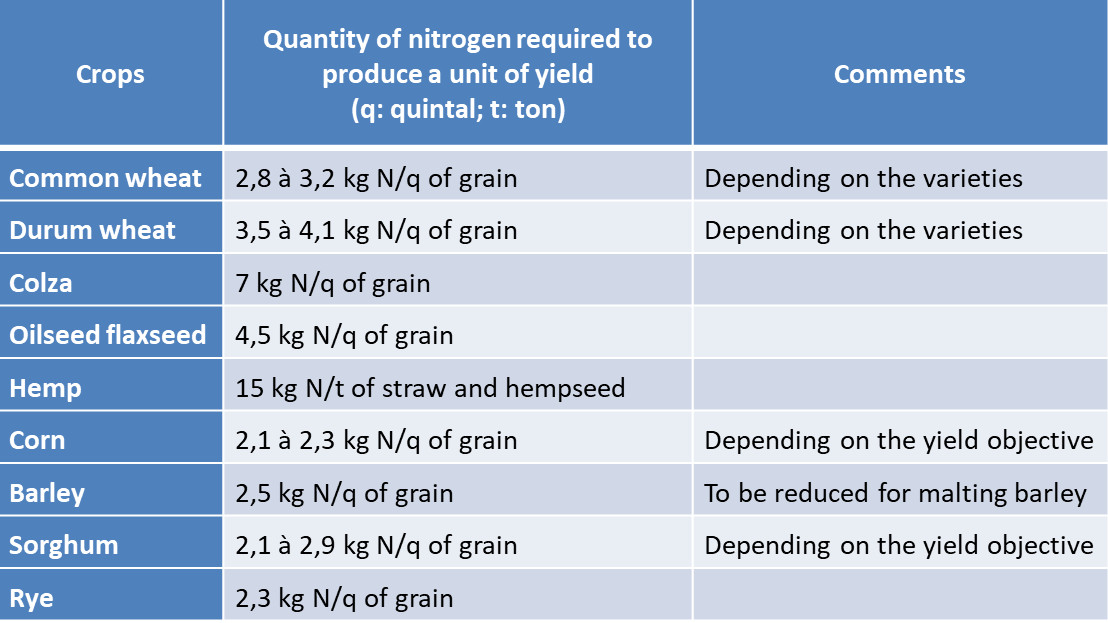
Farmers need to define nitrogen inputs to crops before they develop. One of their difficulties lies in this a priori assessment of nitrogen needs and in the practices to be implemented to ensure that nitrogen availability is in line with vegetation needs. It is important to remember that the development of cultivated plants is strongly dependent on the availability they can have during their growth. The experiment enabled agronomists to define needs ratios for the different crops (Table 1).
Nitrogen inputs to crops are based on estimated needs on the one hand and supplies on the other hand. These estimates established at the beginning of the crop can eventually be adjusted according to the development of the crop, changes in environmental conditions and knowledge of the environment. The quantities to be provided must take into account the nitrogen availability from different sources to which the plants have access:
- Soil supply results from the mineralization of organic matter present in the form of humus, a consequence of the activity of the soil microflora which releases mineral nitrogen almost continuously. The amount produced depends mainly on the stock of organic nitrogen in the soil and its degree of biodegradability. It can vary greatly with soil temperature and humidity. We know how to define the transformation rate constants of this organic stock that make it possible to evaluate this supply.
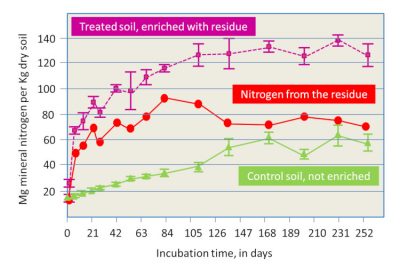
- The supply from organic residues brought to the soil, including crop residues from the past year and possible external inputs: the supply of mineral nitrogen by type of residue can be assessed when they are sufficiently well defined, or from experimental work (Figure 8, [13]). These organic residues include manure and other livestock products, which were the source of nitrogen available before the use of nitrogen fertilizers.
The ways in which crops have been simplified over the past 50 years have led to the separation of crops and livestock on different farms and even in different regions. This has led to a decrease in organic matter inputs to “field crop” soils, resulting in difficulties in maintaining their organic stock. In addition, the concentration of livestock on specialised farms is based on land areas that are often insufficient to properly manage the amount of nitrogen available from animal manure. The need to restore functional links between the two modes of exploitation, crops and livestock, seems essential for good land management. But it seems difficult to implement given the current operating methods adopted.
- the supply of nitrogen from legume crops has probably been the main entry of bioavailable nitrogen from nitrogen gas into agricultural systems over the history of agriculture. These crops allow a synthesis of plant proteins from nitrogen in the air, without going through the nitrate stage, and leave organic residues in the soil that will be a source of mineral nitrogen for subsequent crops.
The ease of use of nitrogen fertilizers, the selling prices of agricultural products and international trade agreements were at the root of a drastic decrease in legume crops in France during the second half of the 20th century. Increases in fertilizer costs and the environmental benefits of biological nitrogen fixation (purchase savings, limitation of greenhouse gas emissions, nitrogen supply from the soil for subsequent crops and improved soil maintenance…) are currently driving the redevelopment of these crops. However, this development is largely dependent on local business opportunities.
- The supply of mineral fertilizers is the supplementary source to supplement crop needs when the sum of other inputs cannot meet the needs. The ease and flexibility of use of nitrogenous mineral fertilizers, together with the observed effectiveness of plant response, were at the origin of the strong development of these fertilizers during the second half of the 20th century.
However, this ease of use has been accompanied by unexpected side effects with selection methods that have probably highlighted crop varieties that preferentially respond to nitrogen fertilization practices. This shows the selection of legumes that develop by assimilating the mineral nitrogen supplied to the soil rather than by associating with a microflora fixing nitrogen from the air. We have also selected wheat varieties that form their grains over a short period of time, the time of availability of this mineral nitrogen supply, rather than varieties with nitrogen nutrition that is more spread over time and enhances the value of the nitrogen provided by the soil… Plant selection is thus influenced by the agricultural practices in place, and therefore increases the difficulty of changing the latter.
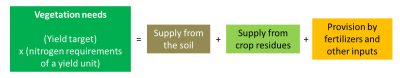
Nitrogen management in our cropping systems using nitrogen fertilizers has been designed on the same conceptual model: to define the volume and dynamics of plant needs and to provide by the fertilizers the quantities of nitrogen necessary to cover these needs in addition to supplies from the soil and crop residues. The forecast nitrogen balance method used in France (Figure 9) is based on this method of reasoning, with the idea of limiting leaks, while implicitly accepting differences related to environmental fluctuations or diagnostic errors.
This balance sheet method has undoubtedly enabled farmers to better reason their nitrogen fertilization, while allowing them to maintain safety margins. Taking into account the different sources of nitrogen supply makes it possible to adjust the necessary doses by taking into account all inputs. But this diversity of contributions can also lead to safety margins for the estimation of each item and to cumulative uncertainties, with the consequence that the final margin calculated on the supplies to be provided raises questions about the value of evaluating the forecast [14]. This tendency, which is heavy in practice, to want to keep considerable room for manoeuvre, probably explains the difficulty of reducing the nitrogen leaks observed in aquatic environments!
However, many farmers are currently faced with increasing production costs that are out of step with their incomes. Aware both of the environmental nuisances linked to their practices, and of the possibilities of different optimal management methods (economic, technical, quality of life), some farmers are seeking to redefine the way their farms operate and to develop closer management of their inputs{ind-text}Products and raw materials used by farmers for agricultural production purposes: seeds, fertilisers, plant protection products, fuels, etc. {end-tooltip}. Some are moving towards organic farming forms, with very specific specifications. Others seek to enhance their knowledge and know-how through the rediscovery of a more traditional agronomy. In terms of nitrogen management, the interest of legume crops is thus being rediscovered, either as a main crop or as a catch cropCrop that generally follows a main crop in the same year and precedes a main crop in the following year. A catch crop can be set up with a harvesting objective or simply with vegetation cover that will be buried before the next main crop. or the ability of crops to undergo moderate nitrogen nutrition stress phases allowing for less fertilization without significant penalty to the final yield of the crop [15]. These approaches to nitrogen management are part of a broader context of research into more economical and environmentally friendly agricultural management methods, with other work on soil management methods and the limitation of the use of plant protection products. With in the background the concern to maintain a sufficient economic margin.
There is currently a development of scientific and techno-economic work aimed at comparing different production methods corresponding to different levels of intensification. From these different management modes, which reflect a real bubbling in the way agricultural practices are approached, new conclusions and practices should emerge [16],[17]. The reasoning behind nitrogen fertilization is part of this evolving context. New strategies are emerging that need to be helped to emerge.
References and notes
Cover image. Eutrophication of an aquatic environment, generally related to excessive nitrogen input, mainly from agricultural nitrates and wastewater [Source: F. Lamiot (CC BY-SA 2.5), via Wikimedia Commons].
[1] Chevassus-au-Louis B., Andral B., Feminas A. & Bouvier M. (2012) Bilan des connaissances scientifiques sur les causes de prolifération de macroalgues vertes – Application à la situation de la Bretagne et propositions. Ministry of Ecology and Sustainable Development and Ministry of Agriculture. 147 p. (in french)
[2] Ministère de l’Environnement, de l’Énergie et de la Mer (2013) Indicateurs de développement durable territoriaux. Les nitrates dans les eaux douces. 5p. Disponible à l’adresse : http://www.statistiques.developpement-durable.gouv.fr/indicateurs-indices/f/1831/1328/nitrates-eaux-douces.html (Consulté en mars 2017) (in french)
[3] Albacore J.C. Philippot L. (2011) Denitrification. In: Handbook of Soil Sciences: Properties and Processes, pp. 32-40. Boca Raton, USA: CRC Press
[4] FAO/WHO (1996) Toxicological evaluation of certain food additives and contaminants. Geneva. World Health Organization, Joint FAO/WHO Expert Committee on food additives (WHO Food Additives Series N°35)
[5] WHO (2011) Nitrate and Nitrite in Drinking-water. Background document for development of WHO. Guidelines for drinking-water quality. 23p.
[6] Leclerc H. (2008) Nitrates de l’eau d’alimentation et risques pour la santé. Acad Agric Fr., 14 mai 2008, 4p. (in french)
[7] Hamon M. (2007) Les nitrates peuvent-ils induire une toxicité indirecte ? Ann. Pharm. Fr., 66, 347-355.
[8] Veena S. Rashmi S. (2014) A review on mechanism of nitrosamine formation, metabolism and toxicity in vivo. Int. J. Toxicol Pharm. Res., 6, 86-96.
[9] Conseil Supérieur d’Hygiène Publique de France (1996) Présentation et discussion d’un document élaboré par le Dr L’hirondel sur des normes et des recommandations concernant les nitrates. CR 13/02/1996. 5p. (in french)
[10] CIPEL (2015) Rapport Eaux du Léman – Campagne 2014, 261p ; Jacquet S. et al (2013) Suivi environnemental des eaux du lac du Bourget pour l’année 2012. Rapport INRA, CISALB, CALB, 226p. (in french)
[11] CITEPA (2015) Changement climatique et effet de serre : https://www.citepa.org. 11p (in french)
[12] Cour de Justice européenne (2014) Arrêt de la cour du 4 septembre 2014. Commission Européenne contre République française. Affaire C-237/12. (in french)
[13] Page S., Hénault C., Chèneby D., Lagacherie B. & Germon J.C. (1998) Devenir de l’azote des eaux résiduaires de féculerie après épandage sur un sol cultivé. Étude et Gestion des Sols, 5, 117-133. (in french)
[14] Germon J.C. (2013) Gestion de la fertilisation azotée en agriculture : enjeux environnementaux et perspectives agronomiques au niveau du territoire français. Cah Agric, 22, 241-248. (in french)
[15] Jeuffroy M.H., Gate P., Machet J.M. & Recous S. (2013) Gestion de l’azote en grandes cultures : les connaissances et outils disponibles permettent-ils de concilier exigences agronomiques et environnementales ? Cah. Agric., 22, 249-257. (in french)
[16] elarue J. & Cochet H. (2011) Proposition méthodologique pour l’évaluation des projets de développement agricole : l’évaluation systémique d’impact. Économie Rurale 323, pp. 36-54. (in french)
[17] Papy F. (2016) Les agricultures du monde face au dérèglement du climat. L’Encyclopédie du Développement Durable, 12p. (in french)
The Encyclopedia of the Environment by the Association des Encyclopédies de l'Environnement et de l'Énergie (www.a3e.fr), contractually linked to the University of Grenoble Alpes and Grenoble INP, and sponsored by the French Academy of Sciences.
To cite this article: GERMON Jean-Claude (January 5, 2025), Nitrates in the environment, Encyclopedia of the Environment, Accessed January 22, 2025 [online ISSN 2555-0950] url : https://www.encyclopedie-environnement.org/en/life/nitrates-in-environment/.
The articles in the Encyclopedia of the Environment are made available under the terms of the Creative Commons BY-NC-SA license, which authorizes reproduction subject to: citing the source, not making commercial use of them, sharing identical initial conditions, reproducing at each reuse or distribution the mention of this Creative Commons BY-NC-SA license.







