靠空气生存的植物

利用空气中的氮能合成基本的生物分子吗?植物能够通过与土壤细菌建立一种互惠互利的关系来实现这一目标。通过一种特定的识别过程,合作伙伴之间建立了一种共生关系。细菌进入根部,皮层细胞分裂。然后形成一个特定的器官,即根瘤,在根瘤中,空气中的氮合成氨基酸,氨基酸再形成蛋白质。这种关系具有重大的经济和环境优势。如此一来就几乎没有必要施用氮肥,并减少了空气和地下水污染。这些靠当下的空气生存的植物还具有重要的营养价值,因此特别引人关注。
1. 氮,植物生长限制因子
氮(N)与碳(C)、氢(H)和氧(O)一样,是生命和生态系统的重要组成部分。它是生命所必需的,因其参与了许多生物分子的组成,如蛋白质、核酸、核苷酸或叶绿素。活性氮主要以硝态氮(NO3-)和氨态氮(NH3)的形式存在于土壤中,可被植物利用。

植物在地球表面的分布是不均衡的:在温带和热带地区较为密集,在极地和沙漠地区则较为稀疏。这是由于植物的生长发育受到许多环境因子的限制。除水源外,活性氮*是植物生长的第二大限制因子(图1)。也是因为此,氮肥在世界农业中被大量使用。。
植物从根部以NO3-或NH3的形式吸收所需的氮。然而,一些物种也能够与海洋或陆生细菌建立联系,这些细菌可以利用空气中的氮:这就是生物固氮。
2. 植物-固氮菌的关系
2.1. 固氮生物的种类
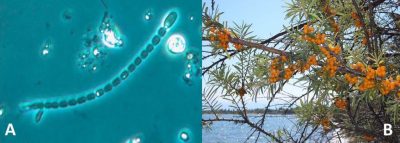
[来源: A,©Jean-Claude Druart, 法国国家农业科学研究院媒体图书馆; B, Vmenkov (GFDL或CC BY-SA 4.0),通过Wikimedia Commons]
大气中氮的生物固定只能通过细菌来实现。固氮细菌可分为三类:
- 海洋蓝藻,包括生活在菌落中的丝状蓝藻 (束毛藻属),游离或与浮游植物共生的单细胞蓝蓝藻(念珠藻属,鱼腥藻属等;图2)。它们占生物固氮量的40-50%。
- 游离土壤细菌,有些是好氧的(氮单胞菌属、固氮菌等),有些是厌氧的(脱硫弧菌属,梭菌属等)。有些被称为光养型,因为它们的能量来源是光(着色菌属,绿菌属等)。另一些则被称为化学营养型,因为它们利用矿物化合物氧化的能量(无机化能营养的:硫杆菌,甲烷球菌属)或有机化合物(有机化能营养的:甲基单胞菌属,固氮菌等)。它们占生物固氮量的5-10%。
- 第三类,这是我们感兴趣的,包括与植物根系共生的土壤细菌*(参见“共生与寄生”)。如弗兰克氏菌属的放线菌,它与各种被子植物( 桤木,沙棘,木麻黄科;图2)和与豆科植物(豆科)共生的根瘤菌类细菌建立了共生关系[1]。该科包括约18000种(大豆,苜蓿,菜豆,小扁豆,落花生,甘草,三叶草,紫藤,含羞草等),特征是蝶形花(蝴蝶状),含有种子的豆荚(花子房的果实),其大多数成员能够通过与根瘤菌的共生利用大气中的氮来产生自己的氮成分。
2.2. 双方如何相互识别?
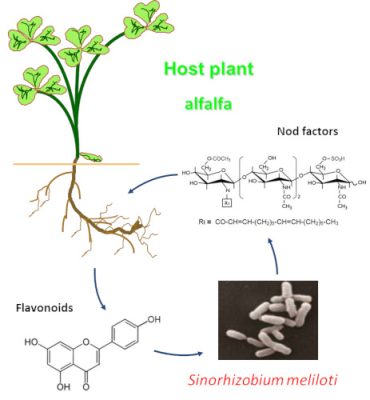
[来源: ©Renaud Brouquisse & Alain Puppo](图3 Host plant 宿主植物,alfalfa 苜蓿,Nod factors 结瘤因子,Flavonoids 类黄酮,Sinorhizobium meliloti 苜蓿中华根瘤菌)
正是通过这两者(植物和细菌)的相互识别,共生过程才开始。为了响应根分泌的类黄酮*(图3),细菌被吸引到根上并合成脂壳寡糖*,又称结瘤因子(用于结瘤)。该过程具有高度特异性:大豆识别的根瘤菌不能被苜蓿识别,反之亦然。
2.3. 结瘤形成
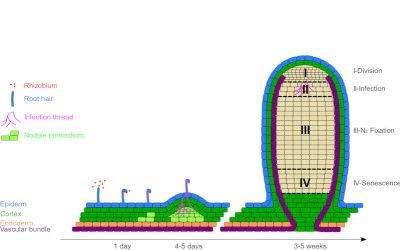
在根表面,受结瘤因子的影响,细菌附着在根毛上,根毛的末端弯曲成“牧羊人十字形”,微共生体*在其中聚集(图4)。根侵染是由组成根毛的细胞质膜内陷引起的。侵染线(一种管状结构)包含进入到根皮层*的细菌。
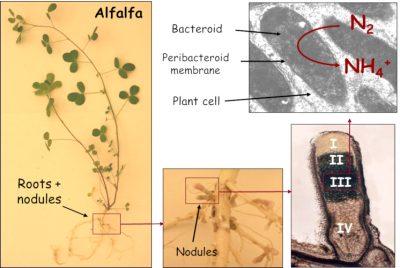
结瘤因子还触发细胞从根皮层去分化(分裂进入),导致在新器官的起源处产生分生组织*:根瘤(图4)。它逐渐被共生细菌(现在称之为类菌体)入侵。然而,它们在植物细胞中并不是游离的;而是被一层植物膜所包围,这层膜叫做类菌体周膜,它会调节两个共生体之间的交换(图5)。
有些无限生长的根瘤保留有分生组织,其结构可分为几个区(图4和图5)[2]: 细胞分裂和结节增加的分裂区(I);细菌进入植物细胞并转化为类菌体的侵染区(II);细菌固氮酶将大气氮(N2)还原为氨(NH3)的固氮区(III); 类菌体,植物细胞死亡的衰老区(IV)。
2.4. 结瘤:类菌体的天堂
在结瘤内,氧浓度远低于大气中的含量。这些微需氧*条件使负责固氮的酶(细菌固氮酶)具有活性。这种酶会被氧灭活。这些条件可以通过两个过程的结合来实现:
- 一方面,由于细胞层没有细胞间隙,结瘤皮层内形成了气体扩散屏障;
- 另一方面,受感染的植物细胞含有一种对氧气有高亲和力的血蛋白:豆血红蛋白。这种蛋白质呈红色,其结构类似于动物肌红蛋白,能以足够低的浓度为类菌体提供氧气,同时不会使固氮酶失活。
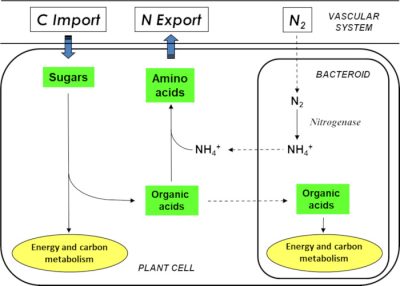
在共生功能中,植物为微生物伙伴提供含碳的营养物质(有机酸),以促进它们的能量代谢;作为交换,类菌体为植物提供氨(NH3/NH4+),与植物蛋白结合(图6)。因为植物能够从大气中吸收合成其生物分子所需的碳(通过光合作用)和氮(通过生物固定),所以“依靠当下的空气生活”这一表述具有完全的意义。
值得注意的是,当豆科植物在天然富含硝酸盐或氨的土壤生长时,它会使用后者作为氮源。共生过程就会遭到抑制,不再发生。
3. 共生关系是生态系统和农学的主要优势
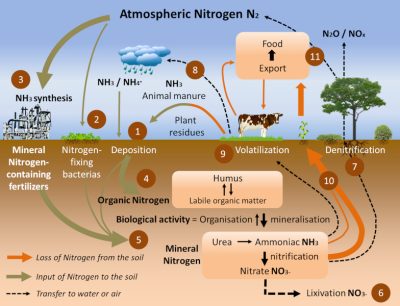
氮通过氮循环永久退出并进入土壤(图7)。主要有三种途径:
- 分解有机物的回收(步骤4)[3]。腐殖质来源于死亡的植物,作物残茬和牲畜粪便,为土壤和水生环境提供有机物(步骤1)。在氧化良好的土壤和水环境中,细菌在硝化过程中将氨转化为亚硝酸盐(NO2-)和硝酸盐(NO3-)(步骤5)。
- 施肥和施氮肥(步骤5)。肥料和氮肥已经通过哈伯-博施化学工艺生产了80多年(图8),该工艺用于在催化剂存在下,用气态氢(H2)使大气气态二氮加氢合成氨(步骤3)。肥料和氮肥现在是北美、南美、欧洲和澳大利亚等工业化国家农业氮的主要来源,近几十年来它们的用量显著增加(图8)。
- 上文提到的生物固氮,特别是通过根瘤菌和豆科植物之间的共生(步骤2)所形成的。豆科植物在地中海盆地种植了至少12000年,几个世纪以来,通过三年的轮作(休耕年,豆类年,谷物年),在农业用地的氮再生方面发挥了重要作用。今天,生物固氮估计每年在1.5亿至2.5亿吨之间,其中约5000万吨来自共生的豆科植物;相比之下,通过哈伯-博施工艺生产的氮肥工业产量约为每年1亿吨[5]。
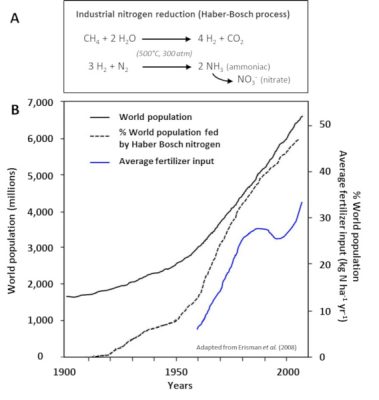
(图8 A Industrial nitrogen reduction (Haber-Bosch process)工业氮还原反应(哈伯-博施法),图8 B World population 世界人口,World population fed by Haber Bosch nitrogen靠哈伯-博施法制氮养活的世界人口,Average fertilizer input平均化肥输入,World population average fertilizer input 世界人口平均化肥输入)
土壤中含氮量和与碳(总氮的97 – 99%)结合的含氮量不等,约为每公顷2 – 10吨。可用于植物生长的氮和氨组分仅占总氮的1% – 3%。从土壤中除氮的过程有三种:
- 通过根系,植物从土壤中吸收硝酸盐和氨,并将它们转化为氨基酸、蛋白质和生长所需的所有氮分子(步骤10)。
- 植物是包括人类在内的草食动物可利用氮的主要来源(步骤9&11 )。
- 在氧含量低的土壤或水生环境中,所谓的“反硝化”细菌将氨和硝酸盐转化为N2,并通过反硝化过程(硝酸盐,NO3–,还原为二氮,N2,还原过程)返回到大气中(步骤7)。
然而,并非土壤中的所有氮都被同化或转化为氮。根据土壤性质和氮化物含量的不同,会发生的变化是:
- 通过径流进入地下水(称为浸出)(步骤6),或以氨或氮氧化物挥发到大气中(NOx:主要是NO和NO2)(步骤8)。
- 这些损失构成土壤氮的耗竭,是大气和地下水的污染源(参见“环境中的硝酸盐”)。
在过去的100多年里,通过工业活动的增加和氮肥的大量使用,人类活动显著地改变了氮循环。今天,地球上活性氮饱和的风险是真实存在的,其对生态系统的影响仍未得到充分的评估。
4. 豆科植物对食物和环境的益处
4.1. 一种食物来源和营养宝库

豆科植物对人和动物的营养具有重要的作用[6]。作为富含蛋白质的饲料,豆科植物可以被用于放牧或饲料储存(干草、青贮、脱水)来喂养动物。豆科植物也可作为动物和人类的食物(图9)。它们富含蛋白质,占其干重的20% – 40%。相比之下,蛋白质只占谷物干重的6% – 13%。此外,豆类蛋白质含有人类无法合成,因此得从饮食中获取的必需氨基酸。豆类蛋白质富含赖氨酸,但含硫氨基酸含量较低(蛋氨酸、半胱氨酸);而谷物蛋白质富含蛋氨酸和半胱氨酸,但赖氨酸含量较低。因此,豆类是谷物的绝佳补充,可实现植物蛋白的均衡饮食[7]。
在大多数的可食用豆科植物中,种子的脂肪含量很低(干重的1%-10%),但也有例外,在一些被称为油籽的品种中,种子的脂肪含量可能占其干重的20%(大豆)甚至50%(花生)。
虽然不同豆科植物的碳水化合物含量差异很大(占其干重的20% – 65%),但它们的血糖指数*通常较低;此外,它们本质上含有一种淀粉(直链淀粉),人体消化缓慢,对血糖的影响很小。此外,种子中的可溶性和不可溶性纤维对消化道健康和消化都有好处;另一方面,一些可发酵纤维(半乳糖苷)可能导致肠胃气胀。
豆类种子还富含维生素(B1、B2、B3、E)和矿物质(钾、磷、镁、锌、铁、锰、钙等)。然而,在干燥的种子中,这些化合物的生物利用率会因抗营养因子(如蛋白酶和淀粉酶抑制剂)或次生代谢物(如植酸盐或单宁)的存在而降低[8]。大多数的抗营养因子被水热处理(种子浸泡和蒸煮)破坏或灭活,从而增加种子各成分的生物利用率。
由于其良好的消化率(浸泡和烹饪后的80% – 90%),豆类蛋白质可以与动物蛋白质相媲美,提供了肉类消费的替代品[2]。因此,就健康和福祉而言,豆类的营养摄入量在多样化和均衡的饮食中占有一席之地。食用它们可以更好地预防心血管疾病、2型糖尿病、肥胖和某些癌症(结肠直肠癌、乳腺癌)[4]。有点缺憾的是,大豆,尤其花生是高度过敏的豆类,食用它们有时会导致食物过敏。
4.2. 氮的来源与环境价值
豆科植物与固氮细菌共生,因此是一种天然肥料。它们是12000多年前在新月沃土被家养的首批植物之一。它死亡后,其根部和地上部分在降解过程中,有机质的矿化作用会释放出各种形式的氮(硝酸盐、氨、氨基酸),这些氮很容易就能被邻近的植物物种或后茬作物吸收。如今,在耕地的氮输入中,豆类种植约占25%(欧洲和北美国家约为20%,亚洲、非洲和南美洲超过50%),工业化肥占63%,燃烧过程占13%[9]。豆科植物的种植对环境有重大影响,因为:
- 它们很少或不需要施肥,可以大大节省较高的肥料生产成本(用哈伯-博施法生产1吨氮肥需要消耗相当于2吨石油)。
- 减少化肥的使用可以通过减少浸出*来减少地下水污染,从而减少富营养化*;
- 它们对温室气体(GHG)排放的平衡有积极影响,包括主要来自矿物肥料和动物粪便的CO2、N2O或氨(共生固定仅产生少量的N2O)。
除了这些与共生存在直接相关的优势之外,豆科植物也有其所固有的优势,无论它们是否与根瘤菌建立了共生关系:
- 通过建立菌根共生关系,它们可以去除土壤中的磷(限制植物生长的另一因素),并使其他植物物种更容易获得磷。
- 它们对土壤有积极的影响(通过根系发育稳定、限制径流和侵蚀),对作物轮作有积极的影响(减少化肥使用、减少植物保护措施)。
- 最后,作为一种天然肥料,它们通过提高植物物种的生产力、吸引传粉者(蜜蜂)和为作物辅助动物和大型动物(鸟类、哺乳动物)提供庇护所而对生物多样性产生积极影响。
5. 作物组合,农业生态的解决方案

法国国家农业科学研究院媒体图书馆; B and C, Christophe Maître, 法国国家农业科学研究院媒体图书馆]
在大规模农业区,法国农业受到作物轮作简化和增加投入(化肥)的显著影响。多样化的农业生态系统日益被认为是可持续发展的重要杠杆。谷物-豆科植物组合、共同播种和收获的模式,集中测试了地块内栽培多样性的提升(图10)。这些组合似乎使有机和传统农业在面对生物和非生物危害时能够应对来自生产、减少投入和作物的环境影响以及稳定方面的挑战[10]。在这种背景下,选择适合这些作物组合的品种成为一项重大挑战。
与单一作物相比,谷类-豆类组合*作物提高了资源利用率。这尤其导致:
- 比单一作物的平均产量更高的粮食/种子产量*,
- 与单独种植豆科植物相比,杂草的生物量减少,
- 与单独种植谷物相比,作物中的蛋白质含量更高。
在不利条件下,作物组合的优点就更加突出:单独种植的每种作物或两种作物的产量低,或单独种植的谷物中蛋白质含量低。当氮限制作物时,组合作物尤其有益,并有助于稳定有机农业的产量。
这些益处的实现与豆科植物在组合种植中的比例密切相关。因此,在草原上,放牧组合的最佳操作条件约为30%-40%饲料豆科饲料,割草组合的最佳操作条件为50%-70%。无论如何,豆科植物都可以成为推动作物转向可持续农业的驱动力。可持续农业尊重生态、经济和社会标准,以确保农业生产的长期发展。
6. 要点
- 一些细菌能够利用大气中的氮来合成其生物分子(海洋蓝藻细菌,游离或与某些植物的根共生的土壤细菌):这就是氮的生物固定。
- 在豆科植物中,共生细菌穿透根系并形成一个新的器官:结瘤。这个器官是将大气中的氮还原为氨的场所;它含有一种与动物肌红蛋白类似的蛋白质:豆血红蛋白。
- 豆类富含蛋白质,是均衡的植物蛋白饮食中谷物蛋白质的极好补充。
- 生物固氮的好处是多方面的:经济方面(节省氮肥)和环境方面(减少空气和地下水污染,促进可持续发展的作物组合)。
参考资料及说明
封面照片:[来源:©Marie-Christine Lhopital, 法国国家农业科学研究院媒体图书馆]
[1] 蔬菜和豆科植物不应该混淆。蔬菜(vegetable)来自拉丁语legumen,指的是果实为豆荚的植物(“legumen”的豆荚)。随后,legumen指所有的蔬菜植物种类。而且在目前的使用中,vegetable指一种可种植的植物,可食用的地方包括种类、叶子、根、块茎、果实、种子;vegetable也指植物可食用的部分。注意,在法语中,豆科植物被称为“légimineuses”。
[2] Ferguson BJ, Indrasumunar A, Hayashi S, Lin M-H, Lin Y-H, Reid DE, Gresshoff PM (2010). Molecular analysis of vegetable nodule development and autoregulation. Journal of Integrative Plant Biology, 52: 61-76.
[3] 括号中的数字为氮气循环图(图7)。
[4] 哈伯-博施法以氨的形式经济地固定大气中的氮,从而可以合成不同的炸药和氮肥。因此,从人口统计学的角度来看,它可能是20世纪最重要的工业过程。
[5] Erisman E, Sutton MA, Galloway J, Klimont Z, Winiwarter W (2008). How a century of ammonia synthesis changed the world. Nature Geoscience, 1: 636-639.
[6] Champ M, Magrini M-B, Simon N, Le Guillou C (2015). Les légumineuses pour l’alimentation humaines : apport nutritionnel et effets santé, usages et perspectives. In Les légumineuses pour des systèmes agricole et alimentaires durables, Schneider A & Huyghe C Coord., Editions Quae, pp 263-295. (法语)
[7] Tomé D (2012). Criteria and markers for protein quality assessment – a review. J. Nutr. 108 (S2): S222-229.
[8] Champ M, Anderson JW, Bach-Knudsen KE (2002). Supplement pulses and human health. J. Nutr, 88 (S3): S237-319.
[9] Cellier P, Schneider A, Thiébeau, Vertès F (2015). Impact environnementaux de l’introduction des légumineuses dans les systèmes de production. In Les légumineuses pour des systèmes agricoles et alimentaires durables, Schneider A & Huyghe C Coord., Editions Quae, pp 297-338. (法语)
[10] Corre-Hellou G et al. (2013). Associations céréale-légumineuse multi-services. Innovations Agronomiques 30, 41-57. (法语)
环境百科全书由环境和能源百科全书协会出版 (www.a3e.fr),该协会与格勒诺布尔阿尔卑斯大学和格勒诺布尔INP有合同关系,并由法国科学院赞助。
引用这篇文章: BROUQUISSE Renaud, PUPPO Alain (2024年3月11日), 靠空气生存的植物, 环境百科全书,咨询于 2025年1月21日 [在线ISSN 2555-0950]网址: https://www.encyclopedie-environnement.org/zh/vivant-zh/plants-that-live-on-air/.
环境百科全书中的文章是根据知识共享BY-NC-SA许可条款提供的,该许可授权复制的条件是:引用来源,不作商业使用,共享相同的初始条件,并且在每次重复使用或分发时复制知识共享BY-NC-SA许可声明。








