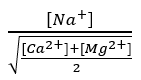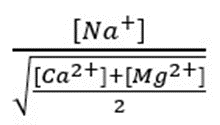Land salinization
PDF1. Soils affected by salinity
Natural environments can be affected by the presence of salts in the soil or water table resulting from the alteration of mineral-rich rocks put in place during geological time. Many areas are thus subject to primary salinization, which develops naturally as a result of the long-term continuous flow of salt-laden groundwater. A number of salt lakes (Figure 2) formed in this way contain ecosystems adapted to these extreme conditions (See Microbes in Extreme Environments).

2. Agricultural soils lost due to salinization
2.1. Secondary salinization
Human activity is responsible for the salinization of soils, which are then made unfit for agriculture. This is known as secondary salinization (Figure 3).

Climate change, overuse of groundwater, increasing use of poor quality irrigation water, massive irrigation in a semi-arid to arid climate zone and lack of soil leaching [3] may intensify this phenomenon of soil salinization.
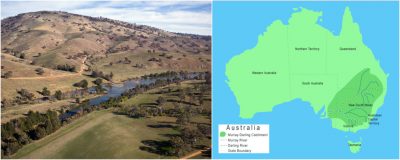
2.2. Example of the Murray-Darling Basin
In south-east Australia, the Murray-Darling Basin, the largest on this continent, is threatened by salinisation. A basin is the area of a country drained by a series of rivers and their tributaries that flow into the sea at a single mouth.
The causes of this disaster are irrefutable: human activities linked to European colonization are responsible for the salinization of the soils of the Murray-Darling Basin (Figure 5):
- The gold rush caused massive deforestation in the area. These forests, by absorbing rainfall, prevented the filling of aquifers, which are groundwater bodies.
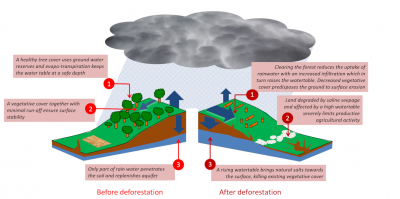
Figure 5. Secondary salinization. Example of the Murray-Darling Basin (Australia). On the left, the diagram illustrates the ecosystem before deforestation, where the forest absorbs rainfall, thus preventing aquifer recharge. After deforestation, the aquifers fill up and come up, causing salt to rise to the surface of the ground (right). [Source: © Murray-Darling Basin Commission] - Deforestation caused a gradual replenishment of shallow aquifers [4].
- Unfortunately, these aquifers are naturally charged with salt. At one or two meters from the surface, water loaded with salts rises up by capillarity.
- Within 150 years, the soil horizons are successively contaminated by salt until they form a deposit on the surface due to evaporation.
2.3. Irrigation & salinity
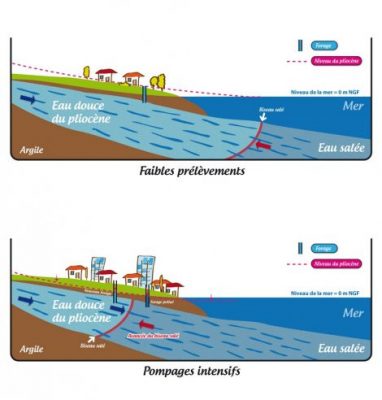
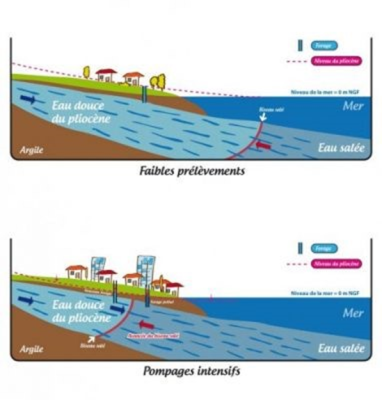
Human activity with the pumping of freshwater into coastal aquifers causes saltwater intrusion that reduces water quality. Figure 6 describes this intrusion:
- reasonable pumping of the water table maintains its level through natural recharge.
- if human activity requires a greater withdrawal, salt water from the sea intrudes into the groundwater table, making it brackish.
2.4. Soil salinity and sea level rise
Finally, soil salinity due to sea level rise, seawater infiltration by waves, wind transport of sea spray or storms causes problems for coastal agriculture. Examples on this subject abound. One example is the problems of salinization of rice fields in the Mekong Delta in Vietnam, which suffer not only from seawater intrusion, but also from the reduction in the flow of this great river caused by the construction of upstream dams that prevent optimal leaching of saline soils. There are also problems of salinization of soils on which vines grow in the Hérault (South of France) [6].
3. Deleterious effects of sodium on soil structure
The sodium (Na+) cation not only impairs plant growth and development, but also causes soil destructuring. These problems, which are increasing worldwide, require agriculture to use soil management techniques to reduce its harmful effects.
Excess sodium (Na+) in the soil changes the physico-chemical properties of the soil. Soil is composed of solid, liquid (water and dissolved elements) and gaseous constituents:
- The solid constituents of the soil are composed of organic matter (resulting from the degradation of plants and animals, dejecta, …) and mineral matter.
- The mineral matter is composed of a coarse fraction (gravel, …) and a fine fraction (clay, …).
- The clays are in the form of particles of less than 2 µm which serve as a “glue” for the larger elements. Thus, clays constitute the mineral colloidal fraction of the soil and give the soil its physico-chemical properties.
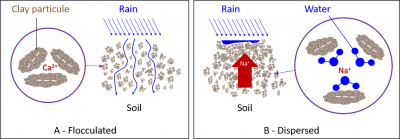
The binding intensity of bivalent cations to clays is higher than that of monovalent cations, mainly due to a higher hydration shell in K+ and Na+ ions. Thus, Na+ provides the least stable flocculation compared to other positive ions.
- Na+ remains mostly in solution, but it can also replace a small proportion of Ca2+ and Mg2+ ions on clays, which is known as the proportion of exchangeable Na+.
- Na+ ion will act with fresh water (during rainfall or irrigation) to form a strong base, significantly alkalizing the soil and producing the hydroxide ion OH–. The combination of the latter with the H+ ion will cause the loss of H+ ions adsorbed on the clays, reducing their flocculation.
The excess Na+ ion in the soil therefore has a strong dispersing effect on clays (Figure 7B).
Dispersed clays produce a compact and asphyxiating soil for the roots, whereas in the flocculated state, clays promote aeration, water permeability and the life of beneficial microorganisms.
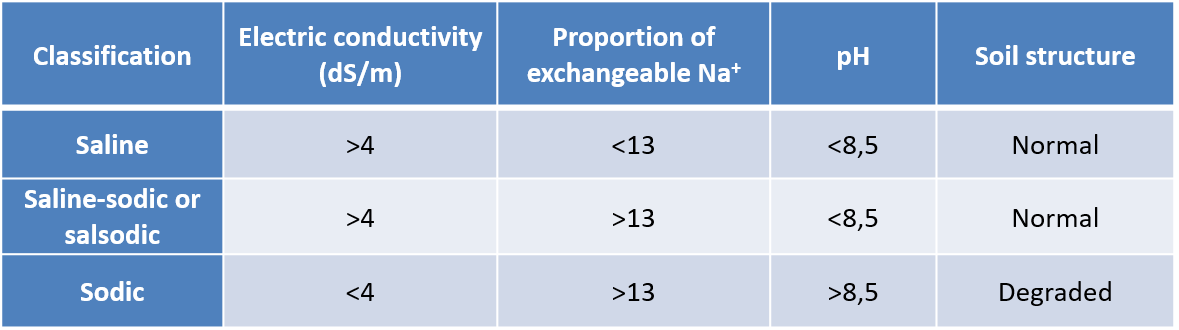
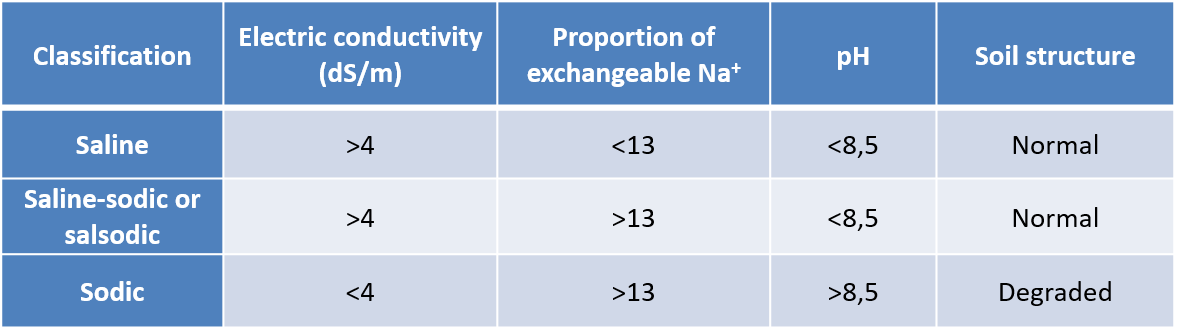
Salinized soils are classified according to:
- their electrical conductivity value (in S/cm) which is related to the salt concentration ;
- their proportion of exchangeable Na+;
- their pH.
The proportion of exchangeable Na+ is closely related to the ratio :
Where the concentrations of Na+, Ca2+ and Mg2+ are expressed in milliequivalents/L; the higher the value, the more Na+ will interfere with the flocculation of the clays.
The classification then includes saline, sodic and saline-sodic soils (Table 1).
Various agricultural practices can limit the harmful effects of excess salt on plant cultivation [7]. When these practices are unfortunately no longer effective, and in particular in the context of saline soils rehabilitation, the removal of salt that has accumulated on the soil surface by mechanical means temporarily improves crop growth.
Flooding the plots with fresh water also helps to desalinate the soil [8]. The supply of fresh water by infiltration into the soil dissolves excess salt and eliminates it if this water is well drained; this is called leaching. This is the most effective procedure for removing salt from the root zone of soils. Drainage is important here to avoid increasing the water table in salt. Other solutions such as adding organic matter to the soil can also be used [9].
Notes and References
Cover image. Aerial view of fields with salt rising to the surface (California Valley). [Source: Scott Bauer / Public domain]
[1] Wicke B, Smeets E, Dornburg V, Vashev B, Gaiser T, Turkenburg W & Faaij A (2011) The global technical and economic potential of bioenergy from salt-affected soils. Energy Environ Sci 4:2669-2681. https://doi.org/10.1039/C1EE01029H
[2] In the European Union, the Mediterranean countries are mainly concerned by this problem (France, Greece, Italy), but also Bulgaria, the Czech Republic, Germany, Hungary, Portugal, Romania and Slovenia. Toth G, Adhikari K, Varallyay G, Toth T, Bodis K & Stolbovoy V (2008) Updated map of salt affected soils in the European Union. In: Toth G, Montanarella, L. & Rusco, E. (ed) Threats to Soil Quality in Europe. Office for Official Publications of the European Communities, Luxembourg, pp 65-77].
[3] Leaching refers to the process by which soluble compounds unsuitable for cultivation are removed from the soil by water. It differs from the term leaching, which refers to non-soluble compounds.
[4] https://www.mdba.gov.au/sites/default/files/archived/mdbc-salinity-reports/2072_Salinity_audit_of_MDB_100_year_perspective.pdf
[5] https://www.nappes-roussillon.fr/L-intrusion-saline.html (in french)
[6] https://france3-regions.francetvinfo.fr/occitanie/vignes-serignan-meurent-intoxication-au-sel-mer-546726.html (in french)
[7] http://www.fao.org/tempref/agl/IPTRID/salinity_brochure_fr.pdf
[8] https://www.mon-viti.com/articles/viticulture/quand-le-sel-ronge-les-vignes (in french)
[9] http://www.fao.org/soils-portal/soil-management/management-of-some-problem-soils/salt-affected-soils/more-information-on-salt-affected-soils/en/
土地盐碱化
PDF1.受盐度影响的土壤
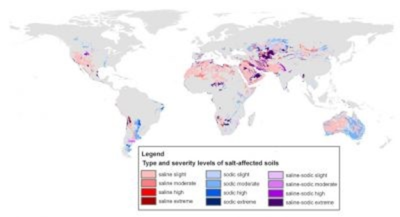
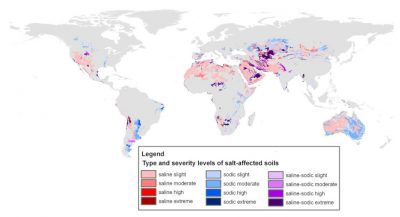
据估计,全世界7%的陆地面积受到盐分的影响。全球受盐影响的土地面积约为1.1 GHa,其中14%被归类为森林、湿地或(国际)国家保护区,由于可持续性问题,被认为无法进行生物量生产(图1)。[1]
自然环境可能会受到土壤或地下水位中盐分的影响,盐分的存在是地质时期沉积的富含矿物质的岩石发生风化的结果。因此,许多地区都受到原生盐碱化的影响,这是含盐地下水长期连续流动的结果。以这种方式形成的许多盐湖(图2)包含适应这些极端条件的生态系统(见极端环境中的微生物)。

盐度主要是氯化钠(NaCl)引起的,氯化钙和氯化镁是最容易溶解的,碳酸盐和硫酸盐对盐度的形成也有小幅度的贡献(见图1)。
2.因盐碱化而流失的农业土壤
2.1.次生盐渍化
人类活动导致土壤盐碱化,从而使土壤不适合农业。这就是所谓的次生盐渍化(图3)

联合国粮食及农业组织估计,世界上20%的灌溉土地受到盐碱问题的影响[2]。全世界每年有1000万公顷的农田被土壤盐碱化破坏。
气候变化、过度使用地下水、越来越多地使用劣质灌溉水、半干旱至干旱气候区的大规模灌溉以及缺乏土壤淋溶[3]可能加剧这种土壤盐渍化现象。
2.2.默里-达令流域示例
在澳大利亚东南部,该大陆最大的默里-达令流域受到盐碱化的威胁。流域是一个地区被一系列河流及其支流穿过,并通过一个河口流入大海的地方。
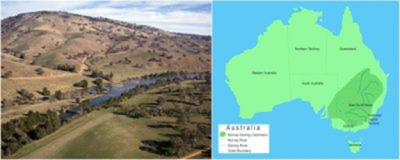
墨累-达令盆地(图4)占澳大利亚陆地面积的1/7,提供了40%的农业生产,养活了300万人。
这场灾难的原因是无可辩驳的:与欧洲殖民有关的人类活动导致了默里-达令流域土壤的盐碱化(图5):
- 淘金热在该地区造成了大规模的森林砍伐。这些森林通过吸收雨水,阻止了蓄水层、地下水体的填充。
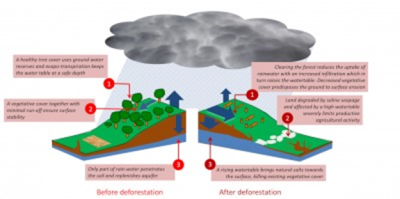
- 森林砍伐导致浅层含水层逐渐补充[4]。
- 不幸的是,这些含水层中自然含有盐。在离地表一两米的地方,含盐的水会引起毛细作用上升。
- 在150年内,土壤层连续受到盐的污染,直到它们由于蒸发而在表面形成沉积物
2.3.灌溉和盐度
在降雨量不足的干旱至半干旱地区,农业离不开灌溉。不幸的是,如果使用含盐含水层中的水进行灌溉,会导致根区含盐量增加。降雨量少或无法用淡水灌溉,这就阻止了利用淋滤去除这些土壤中多余的盐分。因此,灌溉水通过植物的蒸腾和蒸发从土壤转移到大气中,使盐分溶解在土壤中,加剧盐碱化。
人类将淡水泵入海岸含水层的活动导致海水入侵,从而降低水质。图6描述了这种入侵:
- 地下水位的合理抽水通过自然补给维持其水位。
- 如果人类活动需要更大程度的开采,海水会侵入地下水位,使其变得微咸。
2.4.土壤盐分和海平面上升
最后,由于海平面上升、海浪带来的海水渗透、风吹来的海洋飞沫或风暴而造成的土壤盐分给沿海农业带来了问题。关于这个问题的例子比比皆是。一个例子是越南湄公河三角洲的稻田盐碱化问题,这一问题不仅受到海水入侵的影响,而且还受到上游大坝建设导致的河流流量减少而阻止了盐渍土的最佳淋滤。在Hérault(法国南部),种植葡萄藤的土壤也存在盐碱化问题[6]。
3.钠对土壤结构的有害影响
钠离子不仅影响植物的生长发育,而且会破坏土壤结构。这些问题在世界范围内日益严重,因此要求农业使用土壤管理技术来减少其有害影响。
土壤中过量的钠(Na+)会改变土壤的物理化学性质。土壤由固体、液体(水和溶解元素)和气体组成:
- 土壤的固体成分由有机质(由动植物、粪便等降解产生)和矿物质组成。
- 矿物质由粗粒级(砾石等)和细粒级(粘土等)组成。
- 粘土以小于2µm的颗粒形式存在,用作较大尺寸元素的“粘合剂”。因此,粘土构成土壤的矿物胶体部分,并赋予土壤其物理化学性质。
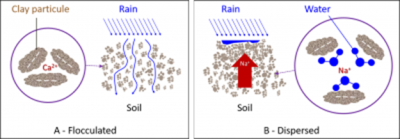
粘土表面带有负电荷;它们自然地相互排斥并保持悬浮状态。在存在质子(酸性介质)或阳离子以中和其电荷的情况下,粘土聚集在一起并沉淀;这称为絮凝(图7)。与H+、K+和Na+不同,阳离子Ca2+、Mg2+和Al3+对粘土具有良好絮凝作用。
二价阳离子与粘土的结合强度高于一价阳离子,这主要是由于K+和Na+离子的水化壳较大。因此,与其他正离子相比,Na+提供的絮凝稳定性最低。
- Na+大部分保留在溶液中,但它也可以替代粘土上的小部分Ca2+和Mg2+离子,即可交换Na+的比例。
- Na+离子与淡水(在降雨或灌溉期间)作用,形成强碱,显著碱化土壤并产生氢氧化物离子OH–。后者与H+离子结合将导致吸附在粘土上的H+离子流失,从而降低其絮凝作用。
因此,土壤中过量的Na+离子对粘土具有强烈的分散作用(图7B)。
分散的粘土为根系产生一种致密的窒息性土壤,而在絮凝状态下,粘土促进通气、透水性和有益微生物的生存。
盐渍土按以下分类:
-
-
- 与盐浓度相关的电导率值(S/cm);
- 交换性Na+的比例;
- 它们的pH值。
可交换Na+的比例与以下比例密切相关:
其中,Na+、Ca2+和Mg2+的浓度以毫当量/L表示;该值越高,Na+对粘土絮凝的干扰越大。
土壤的分类包括盐渍土、碱性土和盐碱土(表1)。
各种农业实践可以限制过量盐分对植物生长的有害影响[7]。不幸的是,当这些做法不再有效时,特别是在盐渍土修复方面,通过机械手段清除土壤表面积累的盐分可以暂时改善作物生长。
用淡水淹没地块也有助于土壤脱盐[8]。通过渗入土壤的方式提供淡水,溶解多余的盐分,并在排水良好的情况下消除盐分;这叫做浸出。这是去除土壤根区盐分最有效的方法。排水在这里很重要,可以避免含盐的地下水位上升。也可以使用其他解决方案,如向土壤中添加有机物[9]。
附注及参考资料
封面图片。盐上升到地表的田地鸟瞰图(加利福尼亚山谷)。[来源:斯科特·鲍尔/公共领域]
[1]Wicke B, Smeets E, Dornburg V, Vashev B, Gaiser T, Turkenburg W & Faaij A (2011) The global technical and economicpotential of bioenergy from salt-affected soils. Energy Environ Sci 4:2669-2681. https://doi.org/10.1039/C1EE01029H
[2]In the European Union, the Mediterranean countries are mainly concerned by this problem (France, Greece, Italy), but also Bulgaria, the Czech Republic, Germany, Hungary, Portugal, Romania and Slovenia. Toth G, Adhikari K, Varallyay G, Toth T,Bodis K & Stolbovoy V (2008) Updated map of salt affected soils in the European Union. In: Toth G, Montanarella, L. & Rusco,E. (ed) Threats to Soil Quality in Europe. Office for Official Publications of the European Communities, Luxembourg, pp 65-77].
[3]Leaching refers to the process by which soluble compounds unsuitable for cultivation are removed from the soil by water. Itdiffers from the term leaching, which refers to non-soluble compounds.
[4]https://www.mdba.gov.au/sites/default/files/archived/mdbc-salinity-reports/2072_Salinity_audit_of_MDB_100_year_perspective.pd
[5]https://www.nappes-roussillon.fr/L-intrusion-saline.html (in french)
[6]https://france3-regions.francetvinfo.fr/occitanie/vignes-serignan-meurent-intoxication-au-sel-mer-546726.html (in french)
[7]http://www.fao.org/tempref/agl/IPTRID/salinity_brochure_fr.pdf
[8]https://www.mon-viti.com/articles/viticulture/quand-le-sel-ronge-les-vignes (in french)
[9]http://www.fao.org/soils-portal/soil-management/management-of-some-problem-soils/salt-affected-soils/more-information-on-salt-a