蝙蝠和冠状病毒的出现
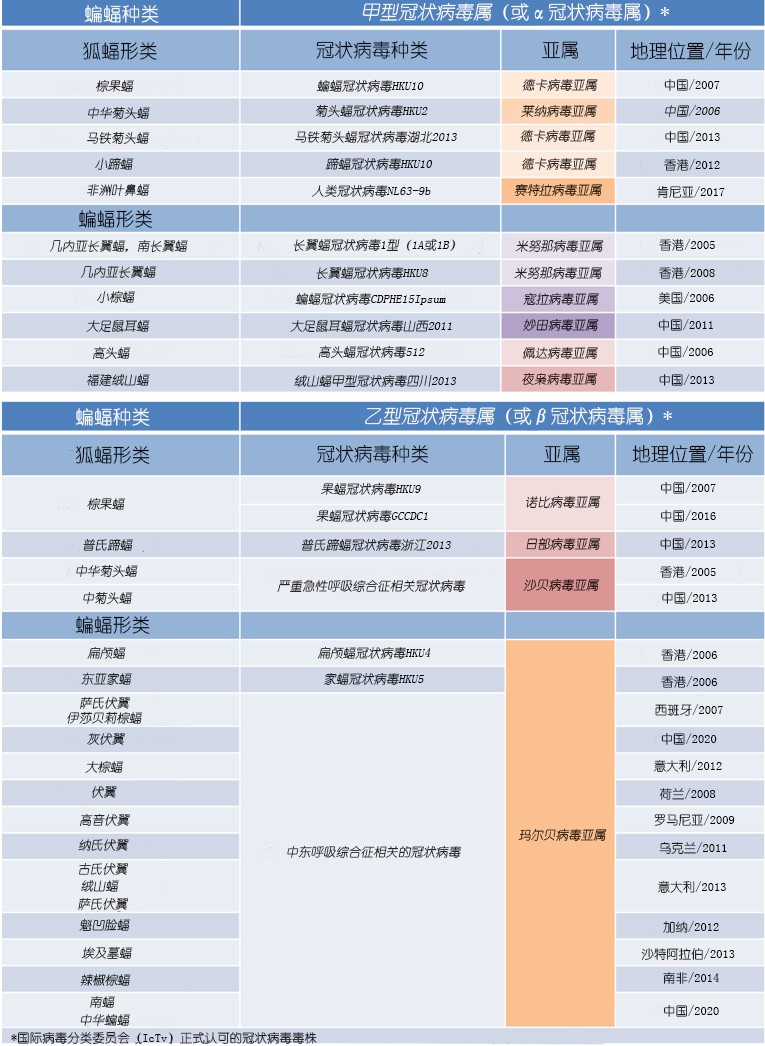
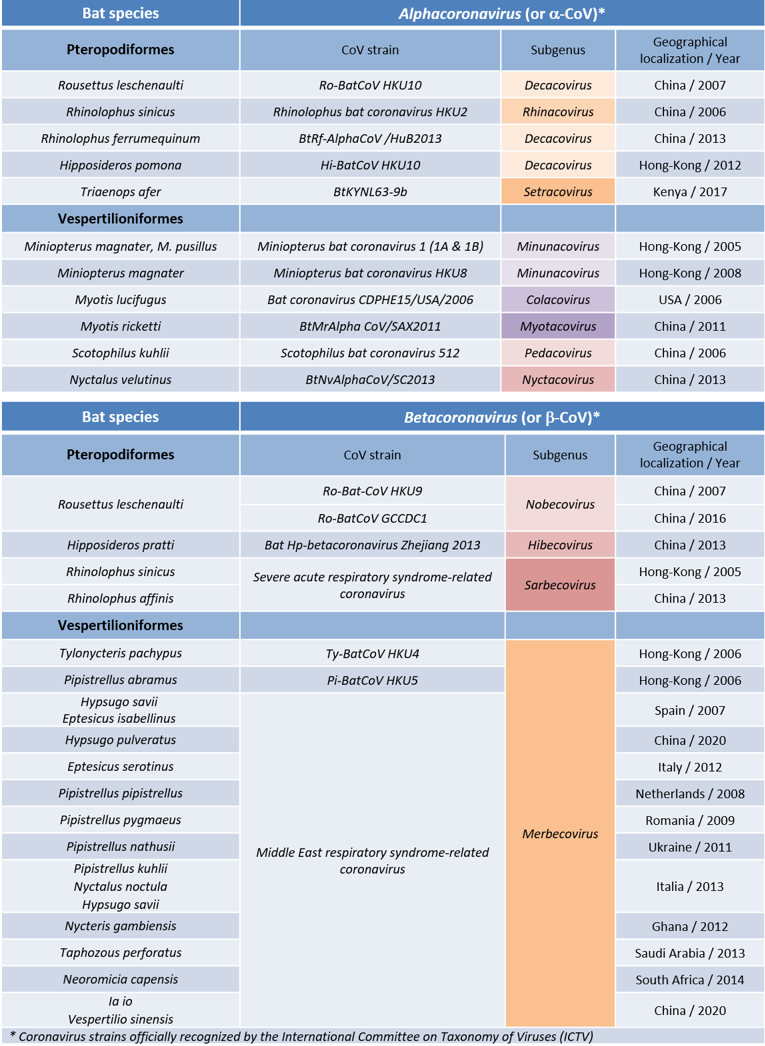
PDF1. 大多数已知的冠状病毒来自于蝙蝠

[图片来源© François Moutou];B,马铁菊头蝠(Rhinolophus ferrumequinum)。[来源:©路易丝-玛丽·普雷奥(Louis-Marie Préau)]
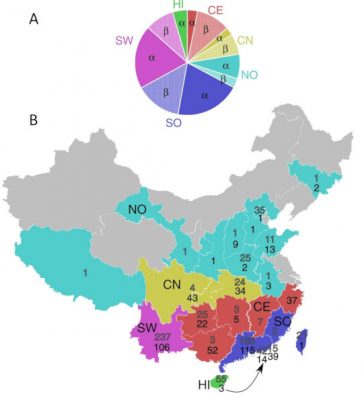
中国是冠状病毒传播的核心区域。这是一个幅员辽阔的国家,其不同的气候导致了蝙蝠和病毒的显著多样性(见表格)。除此之外,蝙蝠与大量人类密切接触,这可能会促使病毒感染人类和家畜。
在2002-2003年爆发的SARS疫情中,果子狸和浣熊(Nyctereutes procyonoides)被确定为冠状病毒(SARS-CoV-1)向人类传播的宿主。很久以后,研究人员才发现,这种冠状病毒起源于菊头蝠属的蝙蝠宿主,包括中华菊头蝠(Rhinolophus sinicus)、马铁菊头蝠(R. ferrumequinum)、中菊头蝠(R. affinis)、大耳菊头蝠(R. macrotis)、单角菊头蝠(R. monoceros),这些病毒的地理来源甚至可以追溯到中国西南部云南省偏远地区的一个洞穴。
表格. 蝙蝠冠状病毒,其中一些是在近期导致流行病的种类。
果子狸和貉在病毒传播链中充当了中间宿主。但野生果子狸的病毒检测结果为阴性,因此很有可能是饲养果子狸、在市场上出售果子狸、在餐馆食用果子狸的各类人类活动造成了野生动物与人类之间的感染。
2. COVID-19是在哪里爆发的?

在新冠肺炎疫情初期,武汉华南海鲜批发市场引起了卫生部门的注意,本以为这家市场只售卖海鲜,实际上这里秘密出售大量活体野生动物。该市场很快被封闭,阻止了进一步的调查。然而少量的采集样本显示,在市场的直接环境(接触面、地面和污水)中存在SARS-CoV-2,但在用以出售的动物中却并不存在。因此,我们必须承认,市场(图2)并不是流行病的罪魁祸首,它只是作为病毒传播的放大器(人流密度大),这里已经有一些感染者了[1]。
零号病人至今未被找到。我们还有机会认识他吗?研究表明,如果新冠疫情的正式日期是2019年的12月底,那么武汉早在2019年的3月就已经有患者了!在法国,2019年11月中旬可能已经出现了COVID-19的病例。我们开始认为人类的第一次感染事件可能要追溯到数年前。研究表明,野生动物和人类间的病毒传播比以前想的要更加频繁,且常常被人们所忽视。就COVID-19而言,这可能是一例独特的从动物到人类的传播事件,且在前段时间大获成功。
3. SARS-CoV-2冠状病毒的起源?
这引发了新的问题:新型SARS-CoV-2冠状病毒从何处起源(图3)[2]。研究人员们以破纪录的速度完成了病毒基因组的测序工作,这个病毒序列被用于和蝙蝠中分离的三种冠状病毒基因组进行比较。
- 两个基因组有97%与对方相同,分别来源于2015年和2017年在浙江省采集的中华菊头蝠(Rhinolophus sinicus)的蝙蝠病毒。这两种病毒有89%与SARS-CoV-2相似[3]。
- 第三个病毒基因组,BatCoV RaTG13与SARS-CoV-2更接近,其序列同源性为2%。该病毒于2013年从与之前的同属蝙蝠身上分离得来,但属于在云南省一个废弃矿井中发现不同物种,中菊头蝠(R. affinis)[4]。
虽然这个同源性的百分比看起来很高,但我们也不能把这两种冠状病毒BatCoV RaTG13和SARS-CoV-2相混淆,它们一定有一个共同祖先,据估计分离时间应在40到70年前[5]。最近,研究人员对2011年在广东地区从列氏果蝠(Rousettus leschenaulti)中分离出来的Ro-BatCoV 和HKU9冠状病毒的基因组重新进行分析,结果发现,重组RaTG13和HKU9这两种病毒会生成一种与SARS-CoV-2非常相似的病毒[6]。在中国南方同一个洞穴里生活的果蝠和菊头蝠很可能交换了冠状病毒,共享了它们的基因序列,从而生成了一种类似于SARS-CoV-2的重组病毒,这并非不可能。
然而,SARS-CoV-2是如何出现在离中国南方较远的湖北武汉的?近年来在湖北的蝙蝠中开展的病毒监测计划未能成功分离出与SARS-CoV相关的病毒,这个问题变得更加扑朔迷离。
4. 致病性的冠状病毒在什么情况下会传播给人类?
4.1. 直接感染?
感染可能直接发生在与蝙蝠有关的狩猎活动中。洞穴中的蝙蝠粪便和气溶胶会感染进入洞穴的狩猎者。在处理或运输蝙蝠尸体或享受野味的时候也可能发生感染。然而,虽然体型较大的果蝠可以称得上是佳肴,但菊头蝠似乎并非如此,它们体型太小了以至于不能成为一道合适的菜品。但也不能排除这种可能性。
血清流行病学研究的支持了病毒直接感染人类的假设,研究表明,大部分在中国南方农村的居民的血液中存在蝙蝠冠状病毒抗体。这些研究结果显示当地居民和洞穴中的蝙蝠间的确发生了病毒的交叉感染[7]。
4.2. 中间宿主?

[来源:丹·贝内特(Dan Bennett),CC BY 2.0,通过维基共享)]
研究人员更接受病毒最终通过一个或多个中间宿主进行传播的假想。在疾病流行初期就有人认为穿山甲起到了中间宿主的作用,但这种说法愈发遭到否定。然而,2019年被中国海关查获的马来穿山甲确实带有一种与SARS-CoV-2[8]受体结合域(RBD)的氨基酸序列非常类似的冠状病毒,可以结合人类的ACE2受体。这就提出了一个问题,即穿山甲和蝙蝠间有何联系。穿山甲是独居动物,冠状病毒会使其脆弱不堪。我们可能有必要接受一个简单的假设,即这些被大量贩运的动物可能在中国的市场被饲养、运输或售卖时,被其他野生动物或走私者感染了(图4)[9]。
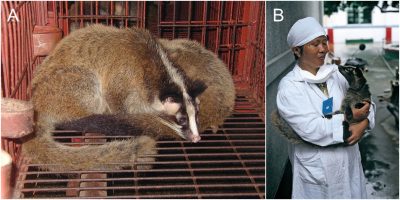
小型食肉动物如果子狸、浣熊会直接食用蝙蝠尸体,它们已经被认为是SARS-CoV-1的携带者了,亚洲獾(Meles leucurus)和鼬獾(Melogale moschata)也有携带病毒的可能。我们还没有排除果子狸[3](图5)这个十分明显的嫌疑者吗?果子狸很受中国人喜爱,它在很长一段时间里被大量养殖,但在2002年的非典疫情之后不得不对其养殖加以控制。当前的养殖场采取了什么措施?我们还知之甚少。
[来源:弗朗索瓦·穆图(François Moutou)]
传统的家畜家禽如猪、鸡和鸭被猜想为其他潜在的中间宿主,但还没有在它们之中观察到病毒传播现象。猫、雪貂和狗等宠物对SARS-CoV-2表现出不同程度的易感性。但因此把它们认为是中间宿主是不令人信服的,尤其是在每个感染病例中,这些动物的主人似乎都是其感染源[10]。
综上所述,目前关于SARS-CoV-2的起源有很多猜测。我们应该知道的是,人们花了15年的时间才识别出了2002年非典疫情的蝙蝠病毒。蝙蝠在COVID-19[11]起源中的作用很有可能在未来的研究中被确认。然而,我们必须知道蝙蝠和病毒之间存在的联系并不新;可能有很长时间了。仅仅因为我们刚刚了解到这种联系,这并不意味着它现在构成的威胁要比以前更大。
5. 结论
- 大多数已知的冠状病毒来自中国的蝙蝠
- 菊头蝠属蝙蝠的冠状病毒引起了SARS。突变使这些病毒直接或通过中间宿主传播给人类。
- 对于导致COVID-19的 SARS-CoV-2,其病毒传播链仍有许多猜测。
- 然而,据我们所知,蝙蝠和病毒新出现的联系并没有构成比以前更大的威胁。
- 为了防止新流行病的出现,人类和野生动物接触的风险因素亟待解决。
感谢弗朗索瓦·穆图(François Moutou)和摄影师路易丝-玛丽·普雷奥(Louis-Marie Préau)(www.louismariepreau.com)对本文的贡献。
参考资料及说明
缩略图:马铁菊头蝠 [来源:©路易丝-玛丽·普雷奥(Louis-Marie Préau),www.louismariepreau.com]
[1] Zhan SH, Deverman BE, Chan YA. (2020). SARS-CoV-2 is well adapted for humans. What does this mean for re-emergence ? bioRxiv preprint : https://doi.org/10.1101/2020.05.01.073262>.
[2] Latinne A, Hu B, Olival KJ, Zhu G, Zhang L, Li H, Chmura AA, Field HE, Zambrana-Torrelio C, Epstein JH, Li B, Zhang W, Wang LF, Shi ZL, Daszak P. (2020). Origin and cross-species transmission of bat coronaviruses in China. Nat Commun 11 (1), 4235. https://doi.org/10.1038/s41467-020-17687-3
[3] Li C, Yang Y, Ren L. (2020). Genetic evolution analysis of 2019 novel coronavirus and coronavirus from other species. Infect Genet Evol, 82, 104285. <doi: 10.1016/j.meegid.2020.104285>.
[4] Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 579 (7798), 270-273. <doi: 10.1038/s41586-020-2012-7>.
[5] Boni MF, Lemey P, Jiang X, Lam TT-Y, Perry BW, Castoe TA, Rambaut A, Robertson DL. (2020). Evolutionary origins of the SARS-CoV-2 sarbecovirus lineage responsible for the COVID-19 pandemic. Nat Microbiol. < doi: 10.1038/s41564-020-0771-4>.
[6] Lau SK, Poon RW, Wong BH, Wang M, Huang Y, Xu H, Guo R, Li KS, Gao K, Chan KH, Zheng BJ, Woo PC, Yuen KY. (2010). Coexistence of different genotypes in the same bat and serological characterization of Rousettus bat coronavirus HKU9 belonging to a novel Betacoronavirus subgroup. J Virol, 84 (21),11385-11394. <Law: 10.1128/JVI.01121-10>.
[7] Li H, Mendelsohn E, Zong C, Zhang W, Hagan E, Wang N, Li S, Yan H, Huang H, Zhu G, Ross N, Chmura A, Terry P, Fielder M, Miller M, Shi Z, Daszak P. (2019). Human-animal interactions and bat coronavirus spillover potential among rural residents in Southern China. Biosaf Health, 1 (2), 84-90. <doi: 10.1016/j.bsheal.2019.10.004. Epub 2019 Nov 9>.
[8] Li X, Giorgi EE, Marichann MH, Foley B, Xiao C, Kong X-P, Chen Y, Korber B, Gao F. (2020). Emergence of SARS-CoV-2 through Recombination and Strong Purifying Selection. bioRxiv. preprint <doi: 10.1101/2020.03.20.000885>.
[9] Lee J, Hughes T, Lee M-H, Field H, Rovie-Ryan J J, Sitam, F T, Sipangkui S, Nathan S K.S.S, Ramirez Diana, Kumar S V, Lasimbang H, Epstein J H, Daszak P. (2020). No evidence of coronaviruses or other potentially zoonotic viruses in Sunda pangolins(Manis javanica) entering the wildlife trade via Malaysia. bioRxiv 2020.06.19.158717; doi: https://doi.org/10.1101/2020.06.19.158717
[10] Leroy EM, Ar Gouilh M, Brugère-Picoux J. (2020). The risk of SARS-CoV-2 transmission to pets and other wild and domestic animals strongly mandates a one-health strategy to control the COVID-19 pandemic. One Health, 100133. <doi: 10.1016/j.onehlt.2020.100133>.
[11] Ar Gouilh M, Puechmaille SJ, Diancourt L, Vandenbogaert M, Serra-Cobo J, Roïg ML, Brown P, Moutou F, Caro V, Vabret A, Manuguerra JC, EPICOREM consortium. (2018). SARS-CoV related Betacoronavirus and diverse Alphacoronavirus members found in western old-world. Virology, 517, 88-97. <doi: 10.1016/j.virol.2018.01.014>.
Bats and the emergence of coronaviruses
PDF1. Most known coronaviruses come from bats

In the case of the SARS outbreak in 2002 – 2003, the masked palm civet and the raccoon dog (Nyctereutes procyonoides) were identified as the animals that transmitted the coronavirus (SARS-CoV-1) to humans. It was only much later that researchers discovered that this coronavirus originated from bat hosts belonging to species of the genus Rhinolophus, including Rhinolophus sinicus, R. ferrumequinum, R. affinis, R. macrotis, R. monoceros, R. pearsoni and R. pusillus (Figure 1). The geographical origin of these viruses has even been traced to a remote cave in Yunnan Province in south-western China.
Table. Bat coronaviruses, some of which are the cause of species responsible for recent epidemics.
Masked palm civets and raccoon dogs thus acted as intermediate hosts between bats and humans in transmission. Since the virus test was negative in civets living in the wild, it is likely that the human activities of raising civets, selling them at markets and eating them in restaurants were responsible for the contamination between wild animals and humans.
2. Where did the COVID-19 outbreak start?

Patient zero is yet to be found. Will we ever know him? If the disease is officially dated at the end of December 2019, analyses show that there will be patients in the Wuhan region as early as March 2019! In France, cases of COVID-19 could have already been present in mid-November 2019. We come to think that the first infection of a human being probably goes back several years. Studies have shown that viral transfers between wildlife and humans are much more frequent than previously thought and often go unnoticed. In the case of COVID-19, this is probably a single animal-to-human transmission event that was successful some time ago.
3. Origin of the SARS-CoV-2 coronavirus?
- Two genomes are 97% identical to each other and originate from bat viruses of the species Rhinolophus sinicus collected in 2015 and 2017 in Zhejiang province. They are 89% similar to SARS-CoV-2 [3].
- The third viral genome, BatCoV RaTG13, is much closer to SARS-CoV-2 with a sequence homology of 96.2%. This virus was isolated in 2013 from a bat of the same genus as before but belonging to a different R. affinis species recovered from an abandoned mine in Yunnan province [4].
Even if this percentage of homology seems high, we cannot confuse the two coronaviruses BatCoV RaTG13 and SARS-CoV-2, which certainly had a common ancestor whose separation is estimated to have occurred 40 to 70 years ago [5]. Recently, by reanalyzing the genome of a coronavirus called Ro-BatCoV HKU9 isolated in 2011 from a Leschenault’s rousette (Rousettus leschenaulti) from the Guangdong region, the researchers were challenged by the fact that by recombining the two viruses RaTG13 and HKU9, a virus very similar to SARS-CoV-2 was released [6]. It is not impossible that rousettes and horseshoe bats living in the same caves in southern China could have exchanged their coronaviruses, which in turn shared sequences of their genomes to produce a recombinant virus similar to SARS-CoV-2.
However, one mystery remains: how did SARS-CoV-2 end up in Wuhan in Hubei Province, which is far removed from the aforementioned provinces in southern China? This mystery is all the more complicated because the viral surveillance campaigns carried out in recent years among Hubei bats have not been able to isolate viruses related to SARS-CoV.
4. What were the circumstances of transmission of the pathogenic coronavirus to humans?
4.1. Direct contamination?
Contamination may have occurred directly during hunting activities involving bats. Hunters enter the caves and can contaminate themselves through contact with the faeces and aerosols generated by them. Contamination may also occur during the handling or transport of bat corpses, or during the cutting up of the bushmeat. However, while large fruit bats are a very popular dish, this does not seem to be the case for horseshoe bats who are too small to make a suitable meal. However, this cannot be ruled out.
The hypothesis of direct transmission of the virus to humans has been supported by seroprevalence studies that have shown the presence of bat coronavirus antibodies in the blood of a significant percentage of people living in rural areas of southern China. These results suggest that exchanges of coronavirus do take place between the inhabitants and bats grouped in colonies in caves near villages [7].
4.2. Intermediate hosts?

Feeding directly on bat corpses could be done by small carnivorous animals such as the Masked palm civet or the raccoon dog, already known as SARS-CoV-1 carriers, but also Asian badgers (Meles leucurus) and Chinese ferret-badgers (Melogale moschata). Haven’t we dismissed an obvious suspect, the masked palm civet [3] (Figure 5)? The Chinese are very fond of this animal and it has long been intensively farmed and had to be brought under control since the SARS epidemic in 2002. What about the measures taken for the current farms? We have little information.
In conclusion, there is a great deal of speculation about the origin of SARS-CoV-2 at this time. It should be remembered that it took 15 years to identify the bat virus that caused the SARS epidemic in 2002. It is highly likely that future discoveries will confirm the role of bats in the origin of COVID-19 [11]. However, it must be understood that these links between bats and viruses are not new; they may be very old. Just because this relationship has just emerged to our knowledge does not mean that it poses a greater threat now than it did before.
5. Messages to remember
- Most known coronaviruses are derived from bats in China.
- The coronaviruses causing SARS originate from bat hosts belonging to the genus Rhinolophus. Mutations have made these viruses contagious in humans after direct transmission or via intermediate hosts.
- For the SARS-CoV-2 responsible for the COVID-19 pandemic in humans, the chain of transmission is still the subject of much speculation.
- However, the newly emerging bat/virus linkages that we know of do not represent a greater threat now than before.
- In order to prevent new epidemics, it is imperative to address the risk factors that expose humans and wildlife.
Thanks to François Moutou and photographer Louis-Marie Préau (www.louismariepreau.com) for their contributions to this article.
Notes and References
Thumbnail photo. Greater horseshoe bat. [Source: © ML Préau, www.louismariepreau.com]
[1] Zhan SH, Deverman BE, Chan YA. (2020). SARS-CoV-2 is well adapted for humans. What does this mean for re-emergence ? bioRxiv preprint : https://doi.org/10.1101/2020.05.01.073262>.
[2] Latinne A, Hu B, Olival KJ, Zhu G, Zhang L, Li H, Chmura AA, Field HE, Zambrana-Torrelio C, Epstein JH, Li B, Zhang W, Wang LF, Shi ZL, Daszak P. (2020). Origin and cross-species transmission of bat coronaviruses in China. Nat Commun 11 (1), 4235. https://doi.org/10.1038/s41467-020-17687-3
[3] Li C, Yang Y, Ren L. (2020). Genetic evolution analysis of 2019 novel coronavirus and coronavirus from other species. Infect Genet Evol, 82, 104285. <doi: 10.1016/j.meegid.2020.104285>.
[4] Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 579 (7798), 270-273. <doi: 10.1038/s41586-020-2012-7>.
[5] Boni MF, Lemey P, Jiang X, Lam TT-Y, Perry BW, Castoe TA, Rambaut A, Robertson DL. (2020). Evolutionary origins of the SARS-CoV-2 sarbecovirus lineage responsible for the COVID-19 pandemic. Nat Microbiol. < doi: 10.1038/s41564-020-0771-4>.
[6] Lau SK, Poon RW, Wong BH, Wang M, Huang Y, Xu H, Guo R, Li KS, Gao K, Chan KH, Zheng BJ, Woo PC, Yuen KY. (2010). Coexistence of different genotypes in the same bat and serological characterization of Rousettus bat coronavirus HKU9 belonging to a novel Betacoronavirus subgroup. J Virol, 84 (21),11385-11394. <Law: 10.1128/JVI.01121-10>.
[7] Li H, Mendelsohn E, Zong C, Zhang W, Hagan E, Wang N, Li S, Yan H, Huang H, Zhu G, Ross N, Chmura A, Terry P, Fielder M, Miller M, Shi Z, Daszak P. (2019). Human-animal interactions and bat coronavirus spillover potential among rural residents in Southern China. Biosaf Health, 1 (2), 84-90. <doi: 10.1016/j.bsheal.2019.10.004. Epub 2019 Nov 9>.
[8] Li X, Giorgi EE, Marichann MH, Foley B, Xiao C, Kong X-P, Chen Y, Korber B, Gao F. (2020). Emergence of SARS-CoV-2 through Recombination and Strong Purifying Selection. bioRxiv. preprint <doi: 10.1101/2020.03.20.000885>.
[9] Lee J, Hughes T, Lee M-H, Field H, Rovie-Ryan J J, Sitam, F T, Sipangkui S, Nathan S K.S.S, Ramirez Diana, Kumar S V, Lasimbang H, Epstein J H, Daszak P. (2020). No evidence of coronaviruses or other potentially zoonotic viruses in Sunda pangolins(Manis javanica) entering the wildlife trade via Malaysia. bioRxiv 2020.06.19.158717; doi: https://doi.org/10.1101/2020.06.19.158717
[10] Leroy EM, Ar Gouilh M, Brugère-Picoux J. (2020). The risk of SARS-CoV-2 transmission to pets and other wild and domestic animals strongly mandates a one-health strategy to control the COVID-19 pandemic. One Health, 100133. <doi: 10.1016/j.onehlt.2020.100133>.
[11] Ar Gouilh M, Puechmaille SJ, Diancourt L, Vandenbogaert M, Serra-Cobo J, Roïg ML, Brown P, Moutou F, Caro V, Vabret A, Manuguerra JC, EPICOREM consortium. (2018). SARS-CoV related Betacoronavirus and diverse Alphacoronavirus members found in western old-world. Virology, 517, 88-97. <doi: 10.1016/j.virol.2018.01.014>.