Improving photosynthesis?
PDFDifferent genetic approaches have been and are being considered to improve the photosynthetic metabolic capacities of C3 crops with a view to adapting them to future climate variations. There is also work leading to industrial applications, such as the search for new energy sources and new bioproducts and biomaterials.
1. Introduction of bacterial pathways for recycling photorespiration products in higher plants

American and German researchers have observed that some photosynthetic microorganisms have three pathways of P-glycolate metabolism, the glycolate pathway followed by CO2 loss that is common to plants and bacteria, and two other pathways specific to bacteria that metabolize P-glycolate but do not emit CO2 external to the leaf tissue. These last two pathways have not been transmitted to plants through evolution. It was then tempting to introduce them into the plants. This was done, not without difficulty, by transferring by genetic engineering these two specifically bacterial metabolic pathways into C3 type plants, tobacco in particular. Processed tobacco plants showed 20-40% higher photosynthetic activity compared to unprocessed C3 plants, which is a great technical achievement. Finally, the trial was also “transformed” in the field (Figure 1), which was not a foregone conclusion. Now these researchers are trying to confirm these results and replicate them on field crops and forest plants. [1]
2. Creating new metabolic pathways for a more efficient CO2 fixation?
Since the improvement of RubisCO performance by chemical means using products that inhibit the enzyme’s oxygenase function and by genetic means has failed, German-Swiss (Max Planck Institute) and American researchers from Thomas Schwander’s team have attempted to improve the carboxylation system. In this way, they have sought and selected the most active carboxylases in the living world and, by modifying them if necessary by genetic engineering, have increased their performance. [2] These “new artificial carboxylases”, together with the enzymes associated with their functioning, when placed in suitable biological environments, showed activities 5 to 20 times higher than those present in “natural” metabolic chains. These researchers have thus created a new carboxylation pathway that was named “CETCH cycle” by the research teams, after the enzyme chain that composes it. [3] They observed that this novel pathway functioned in vitro in non-photosynthetic bacterial cells, while consuming fewer energetic molecules (NADPH and ATP) for their function than the natural photosynthetic RubisCO /Benson, Bassham and Calvin Cycle pathway. All that remains is to introduce this cycle into photosynthetic green cells.
3. Can C4 metabolism be introduced in C3 plants? The case of rice

If the enterprises of genetic transformations of C3 plants into C4 plants are not easy, researchers do not despair of succeeding, especially since we know that the C4 system has appeared several times during evolution in certain families of plants such as amarantaceae for example. It’s a huge challenge. Major plant biology consortia (IRRI, International Rice Research Institute) are involved in this research, as rice is one of the plants most directly consumed by humans. [4]
4. Hydrogen production by microalgae
5. Towards the artificial or bionic leaf
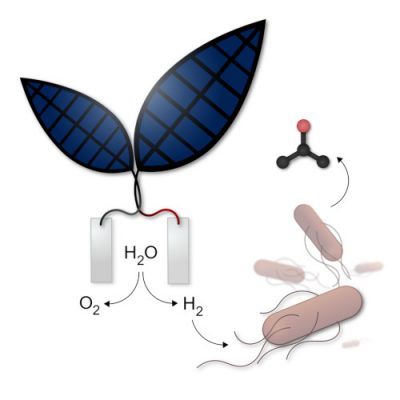
A first type of “artificial leaf”, consisting of an assembly of thin layers of different metals, produces oxygen and hydrogen, the latter of which can be used to operate a fuel cell and thus generate electricity. Professor Nocera, a researcher at Harvard University in the United States, announced that the new artificial leaf could achieve photosynthesis ten times more efficiently than the natural process of photosynthesis (Figure 4). [6] The system would be mature enough to consider commercial applications.
Strano, from MIT (Massachusetts Institute of Technology) and his collaborators have inserted cerium oxide nanoparticles into isolated chloroplasts in order to trap free oxygen radicals during oxidative stress (e.g. strong illumination) and to prolong the photosynthetic process under the best conditions of activity. In a similar vein, Dr. Strano and his collaborators have also introduced DNA-coated carbon nanotubes (powerful prosthetic photo-absorbers) that are negatively charged in isolated chloroplasts. [7] This technique increases the range of wavelength spectrum perceptible by chloroplasts, which thus absorb nearly 50% more light radiation from ultraviolet to near infrared than isolated chloroplasts without this technology. In addition, these researchers have shown that nanotubes present in chloroplasts also detect and sequester nitrogen monoxide NO, one of the gases most involved in air pollution. The researchers reproduced these experiments on whole plants, Arabidopsis thaliana in particular.
Notes and References
[1] Maurino V.G. & Peterhansel C. (2010) Photorespiration: current status and approaches for metabolic engineering. Cur. Op. Plant Biol. 13(3):248-255; Ort D.R., Merchant S.S., et al (2015). Redesigning photosynthesis to sustainably meet global food and bioenergy demand. D.D.A. Natl. Acad. Sci. U.S.A. (28):8529-8533.
[2] Schwander T., von Borzyskowski L.S., Burgener S., Cortina N.S. & Erb T.J.2016. A synthetic pathway for the fixation of carbon dioxide in vitro. Science 354:900-904.
[3] CETCH: crotonyl-CoA/ethylmalonyl-CoA/hydroxybutyryl-CoA
[4] von Caemmerer S., Quick W.P. & Furbank R.T. (2012). Development of C4 Rice: Current Progress and Future Challenges. Science 336:1671-1672.
[5] Desplats, C., Mus F., Cuine S., Billon E., Cournac L. & Peltier G. (2009). Characterization of Nda2, a Plastoquinone-reducing Type II NAD(P)H Dehydrogenase in Chlamydomonas chloroplasts. J. Biol. Chem. 284(7):4148-57.
[6] Liu C., Colón B.C., Ziesack M., Silver P.A. & Nocera D.G. (2016). Water splitting-biosynthetic system with CO2 reduction efficiencies exceeding photosynthesis. Science 352:1210-1213.
[7] Giraldo JP, Landry MP, Faltermeier SM, McNicholas TP, Iverson NM, Boghossian AA, Reuel NF, Hilmer AJ, Sen F, Brew JA & Strano MS (2014).Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nature Materials 13:400-408.




